Sulphur 399 Mar-Apr 2022
31 March 2022
Decreasing CO2 footprint with clean energy from sulphuric acid production
EMISSIONS REDUCTION
Decreasing CO2 footprint with clean energy from sulphuric acid production
Ricardo L. Sepulveda of PegasusTSI reviews options to decrease the CO2 footprint of a fertilizer industrial complex and illustrates the technical and economic feasibility of utilising clean energy from a sulphuric acid plant in a fertilizer complex to produce green hydrogen, which in turn can be used to produce green methanol or green ammonia.
Emissions of carbon dioxide(CO2) and other greenhouse gases are one of the main drivers of climate change, and their reduction presents one of the most urgent challenges today. To slow down the global temperature increase, it is necessary to stabilise the CO2 concentration and other greenhouse gases present in the atmosphere. But currently, far from stabilising their concentrations, greenhouse gases continue to accumulate.
To stabilise, or even reduce CO2 concentrations in the atmosphere, the world needs to achieve net zero emissions. This requires large and rapid emission reductions (Fig. 1).
What is a green technology?
Green technology is a technology that is considered friendly to the environment based on its production process or its supply chain. One example is the hydrogen produced from renewable/clean electricity by water electrolysis.
The production of methanol using green hydrogen and the capture of CO2 allows the decrease in carbon emissions in an industrial complex while generating green methanol.
Ammonia production using green hydrogen (H2) and nitrogen (N2), allows a decrease in carbon footprint in an industrial complex while generating green ammonia.
Methanol Production
Methanol is a valuable chemical with various uses, either as a hydrogen storage compound, a fuel, or as raw material to synthesise other chemical substances.
Conventionally, methanol is produced on an industrial scale from synthesis gas, a combination of varying amounts of H2 , CO, and CO2 frequently derived from gasified coal or steam reforming of natural gas, which have an emission factor between 0.4 to 3.0 t CO2/t methanol
In recent years, interest in methanol production from CO2 has been growing, based on the concept of power-to-fuel. Specifically, power-to-methanol technology is based on the electrochemical separation of water into H 2 and O 2 with the subsequent catalytic hydrogenation of CO2 to liquid methanol.
CO2 hydrogenation to methanol is environmentally friendly for decreasing CO2 emissions. The industrial implementation has been limited mainly due to the higher costs associated with capturing CO2 and producing H2 from clean/renewable energy compared to production from synthesis gas. However, in recent times, it has become necessary to consider new processes that incorporate environmentally beneficial features.
Ammonia production
Ammonia is critical in the manufacturing of fertilizers and is one of the largest-volume synthetic chemicals produced in the world. Conventionally, ammonia is produced by converting hydrocarbons, (NG, LPG, naphtha), into hydrogen (via steam reforming), and combining it with nitrogen, which will have an emission factor of ~2.1 t CO2/t NH3 . Fig. 2 shows the US ammonia supply/disposition balance.
Fertilizer industrial complex
In the following sections options to decrease the CO2 footprint in a fertilizer industrial complex are reviewed with reference to the following processes:
- sulphuric acid energy recovery;
- phosphoric acid production process/ CO2 capture;
- ammonia consumption.
Sulphuric acid energy recovery
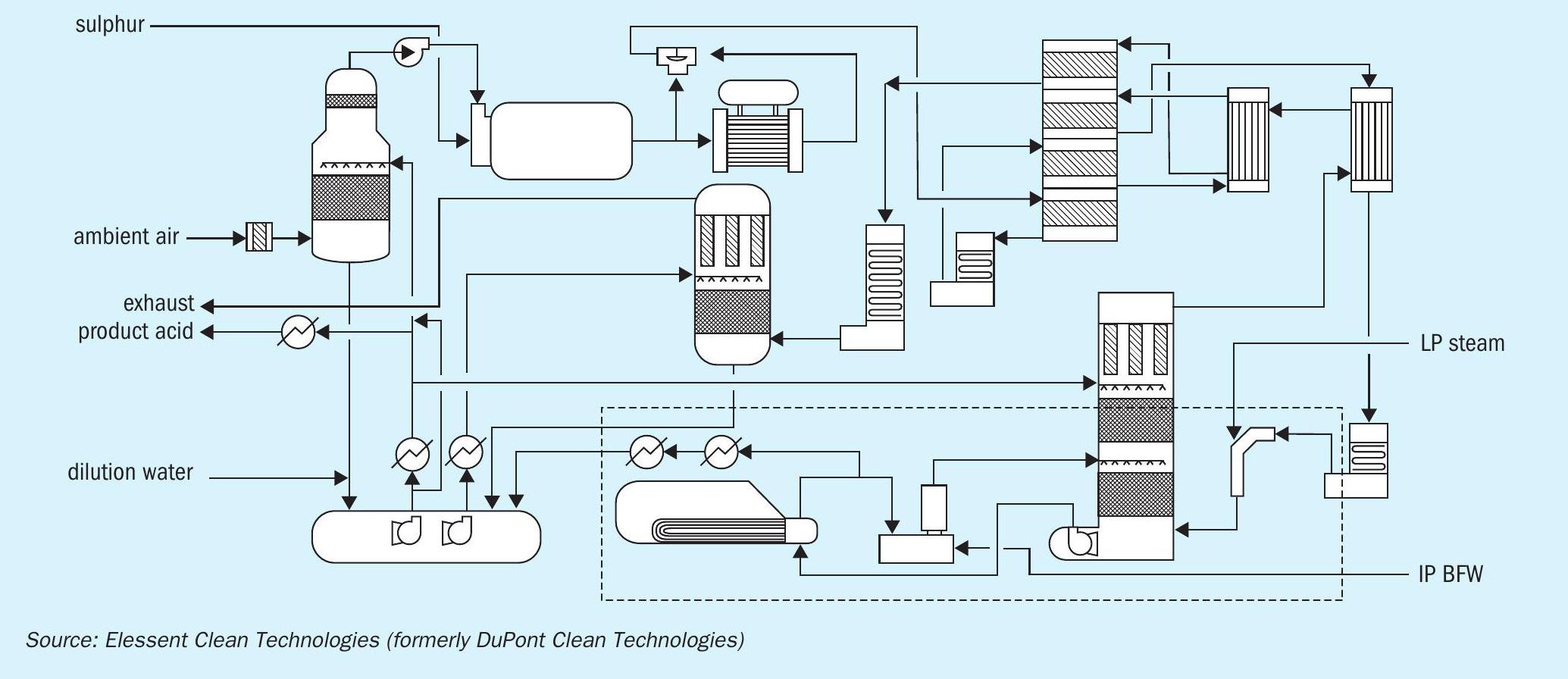
In a fertilizer industrial complex, sulphuric acid is used in the production of phosphoric acid. A by-product of sulphuric acid production is high-pressure steam which can be used for electric power generation and to provide a heat source for fertilizer production. New developments in optimising sulphuric acid plants, like the addition of heat recovery systems (HRS) to existing double contact double absorption plants, provides additional medium-pressure steam generation to allow the production of clean electricity in a condensing steam turbine.
Clean electricity can be used in an electrolyser for the electrolysis of water that separates hydrogen (H2) and oxygen (O2) from water (H2O) to produce green hydrogen, which can be used as a raw material to produce green chemicals.
Fig. 3 shows the MECS® HRS™ technology, which captures and re-uses waste heat from the process and converts it to medium-pressure steam.
For example, a 3,400 t/d sulphuric acid plant can generate 150,000 lb/hr (68 m3 /h) of medium-pressure steam, considering two HRS with a total capacity of 300,000 lb/hr (136 m3/h), the potential electric production in a condensation steam turbine generator will be 24 MW of net electric clean power.
This clean energy will be the source for the production of green hydrogen in the fertilizer industrial complex.
Phosphoric acid production process/ CO2 capture
Carbon dioxide is emitted when the limestone component (CaCO3 ) of phosphate rock reacts with sulphuric acid (H2SO4 ). Process emissions from phosphoric acid production in the US totalled 1.17 million tonnes CO2 per year. According to the US EPA (Technical Support Document for the Phosphoric Acid Production Sector: Proposed Rule for Mandatory Reporting of Greenhouse Gases, 2009) phosphoric acid plants have an average emission factor of 0.15 t CO2 /t phosphoric acid.
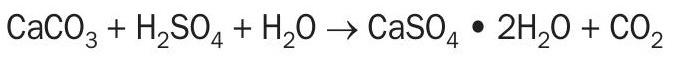
A typical facility with a capacity of 1 million t/a of phosphoric acid will therefore have an estimated total CO2 emission of 150,000 t/a.
For the production of green methanol, it is necessary to have a source of CO2 . The CO2 from the flue gas of the phosphoric acid reactor could be captured, filtered, and concentrated to be used for the production of green methanol.
The production of methanol using green hydrogen and the capture of CO2 , decreases the carbon emission in a fertilizer complex while generating green methanol.
Ammonia consumption
The use of ammonia in fertilizer production e.g., the production of granular mono ammonium phosphate (GMAP) increases the CO2 footprint of the fertilizer complex further due to the ammonia CO2 footprint (2 t CO2 /t ammonia, based on steam reforming of natural gas).
A typical facility with a capacity of 1 million t/a of GMAP will need 130,000 t/a of ammonia which will create a total CO2 footprint of 260,000 t/a. A partial replacement of the ammonia with green ammonia will decrease the CO2 footprint.
Green hydrogen production
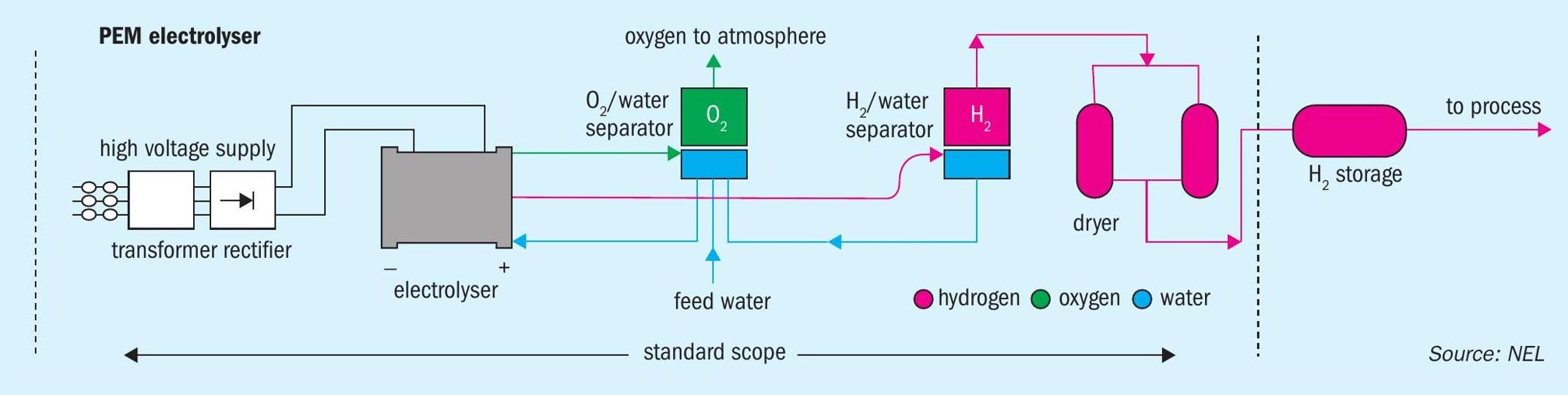
To obtain H2 from the electrolysis of water, at an industrial level, there are two technologies available: alkaline electrolysis (AEL), with a temperature and pressure below 80°C and 30 bar, and proton exchange membrane electrolysis (PEM), with temperature and pressure lower than 100°C and 200 bar (Fig. 4).
AEL technology typically has a lower capital investment (generally uses nickel catalysts) but is less efficient. On the other hand, a comparable PEM technology unit will have a higher capital investment but is more efficient and can operate at higher current densities. Lower hydrogen production costs can be achieved at higher hydrogen production capacities.
In both cases, the dissociation of the water molecule occurs by an electric current applied to two electrodes separated by the electrolyte or a membrane that only allows the passage of positive ions, producing hydrogen at the cathode and oxygen at the anode.
A theoretical 100% efficient electrolyser would consume 39.4 kWh/kg H2 (142 MJ/ kg, 12.75 MJ/m³). This electrical consumption value increases due to system inefficiencies and so for commercial applications it is around 55 kWh/kg H2 .
Generally, the hydrogen produced from electrolysis is for immediate use applications such as oxygen flares or when high purity hydrogen or oxygen is desired.
High pressure electrolysis (HPE) of water produces hydrogen at around 120200 bar at 70°C. Pressurising the hydrogen in the electrolyser eliminates the need for an external hydrogen compressor; the average energy consumption for internal compression is around 3%.
CO2 recovery
A large portion of carbon emitted to the earth’s atmosphere every year is in the form of gaseous CO2 , and approximately 30% of this CO2 comes from fossil fuel power plants.
In addition to rising levels of atmospheric CO2 , the earth’s temperature is increasing. Since CO2 can act as a trap for heat, the reduction of CO2 emissions is an essential area of research.
Separation and recovery of CO2 are near-term goals for emissions reduction. Methods to obtain CO2 from flue gas streams include absorption using solvents or solid sorbents, pressure- and temperature-swing adsorption using various solid sorbents, cryogenic distillation, membranes, among others.
The most promising current method for CO2 separation is liquid absorption using monoethanolamine (MEA). While this method is currently the most widely adopted, the development of ceramic and metallic membranes for membrane diffusion should produce membranes which are significantly more efficient at separation than MEA absorption.
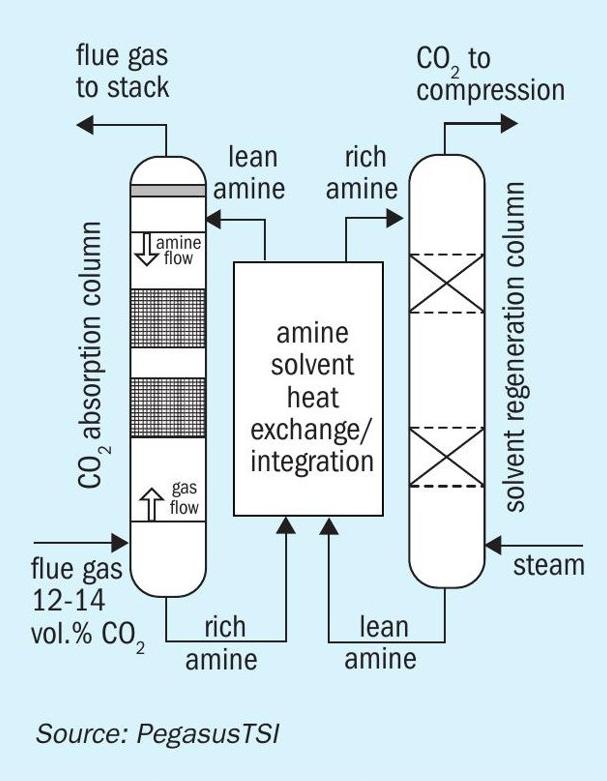
Liquid absorption
The gas stream enters an absorber reactor and flows countercurrently to a CO2 -lean solvent into which CO2 is absorbed and reacts with the MEA to form water-soluble compounds. The treated gas is discharged to the atmosphere and the CO2 -rich solution is pumped to a stripper reactor for regeneration (Fig. 5). In the stripper, the CO2 -rich solution is heated in order to break down the salt and regenerate the MEA solvent. A reboiler provides the heat for regeneration of the MEA solvent in the stripper. Consequently, CO2 is released, producing a concentrated stream which exits the stripper and is then cooled and dehumidified in preparation for compression, transport, and storage. From the stripper, the CO2 -lean solution is cooled and returned to the absorber for reuse. Usable MEA solution concentrations are typically limited by viscosity and corrosion. Therefore, current systems use only between 20% and 30% MEA, with the remaining being water. Although the water present in the solution helps control the solvent temperature during absorption, which is an exothermic reaction, the water also requires significant amounts of sensible heating and stripping energy upon CO2 regeneration.
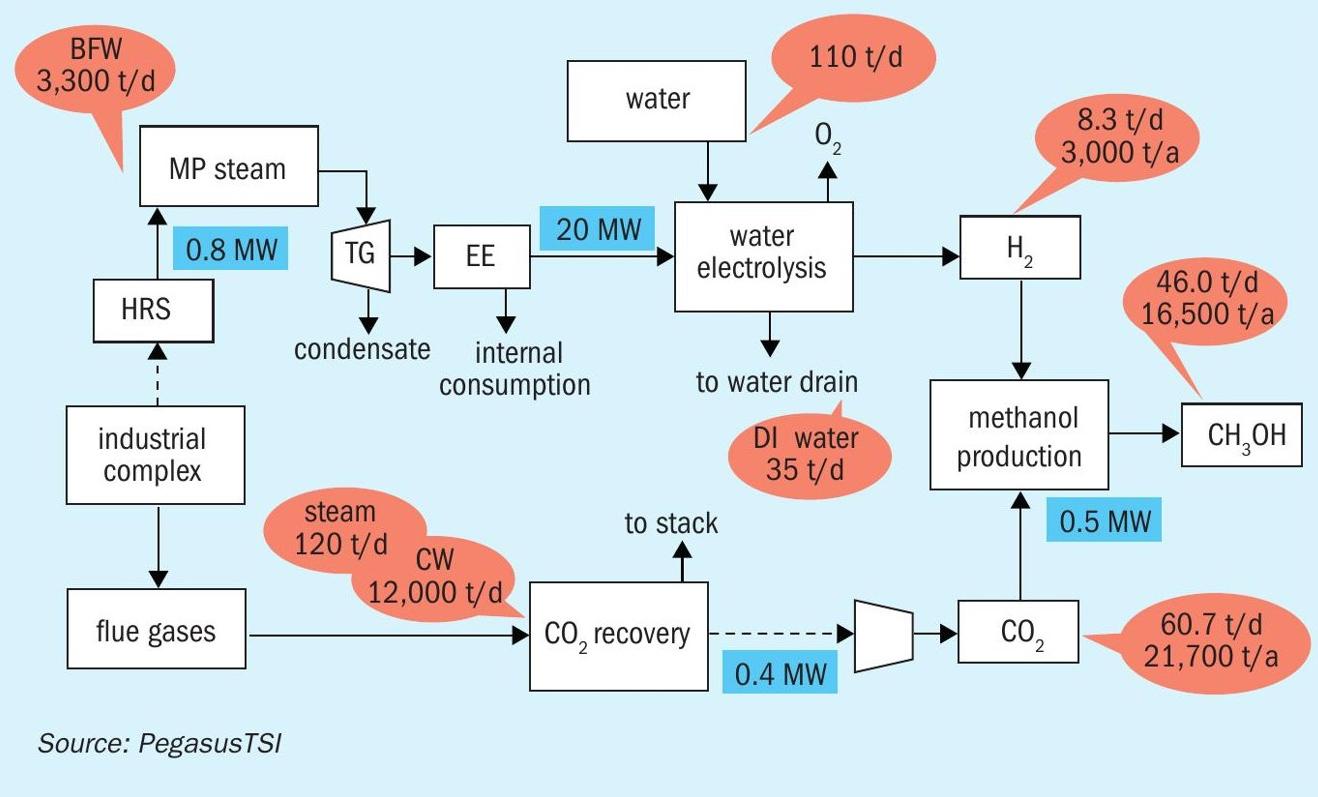
Industrial case for a fertilizer complex
As previously described, an industrial fertilizer complex offers good potential for the production of clean energy coming from the sulphuric acid production energy recovery, which will generate medium pressure steam that can be used to produce clean electricity.
Considering the installation of HRS recovery systems in two sulphuric acid plants of 3,400 t/d, it is possible to generate an additional 300,000 lb/hr (136 m3 /h) of medium-pressure steam, which can be used in condensation steam turbine generator to generate 24 MW of net electric clean power.
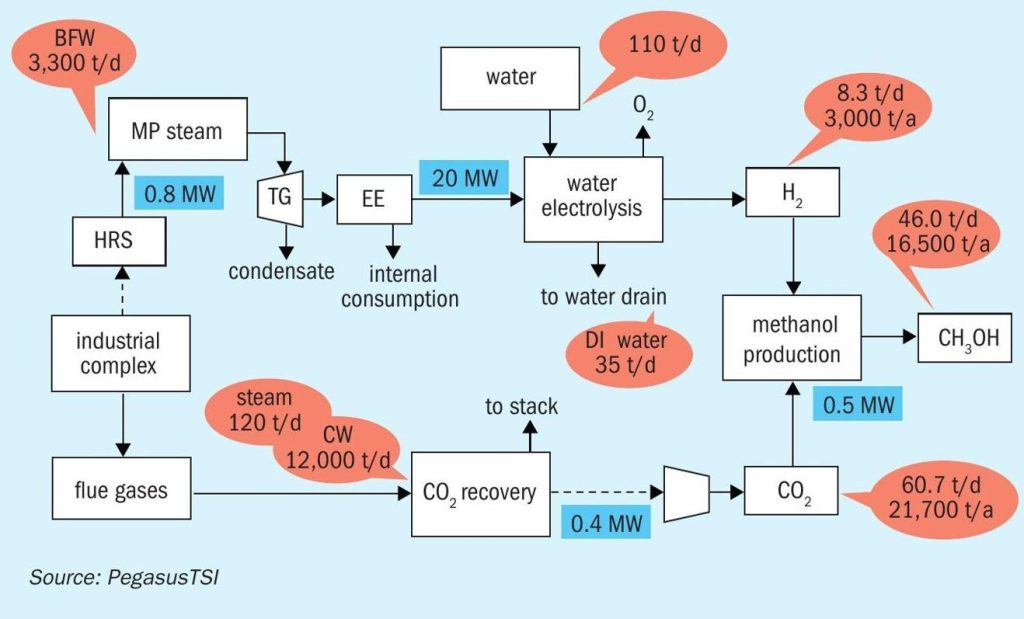
The potential production of hydrogen from a PEM electrolyser with 24 MW is 8.3 t/d of H2 (3,000 t/a).
Carbon dioxide can be recovered from the phosphoric acid reactor flue gas in the phosphoric acid plant to decrease the CO2 footprint of the fertilizer production and for the production of green methanol. In this case 21,700 t/a of CO2 could be recovered, which could react with the 3,000 t/a of hydrogen to produce 16,500 t/a of green methanol (Fig. 6).
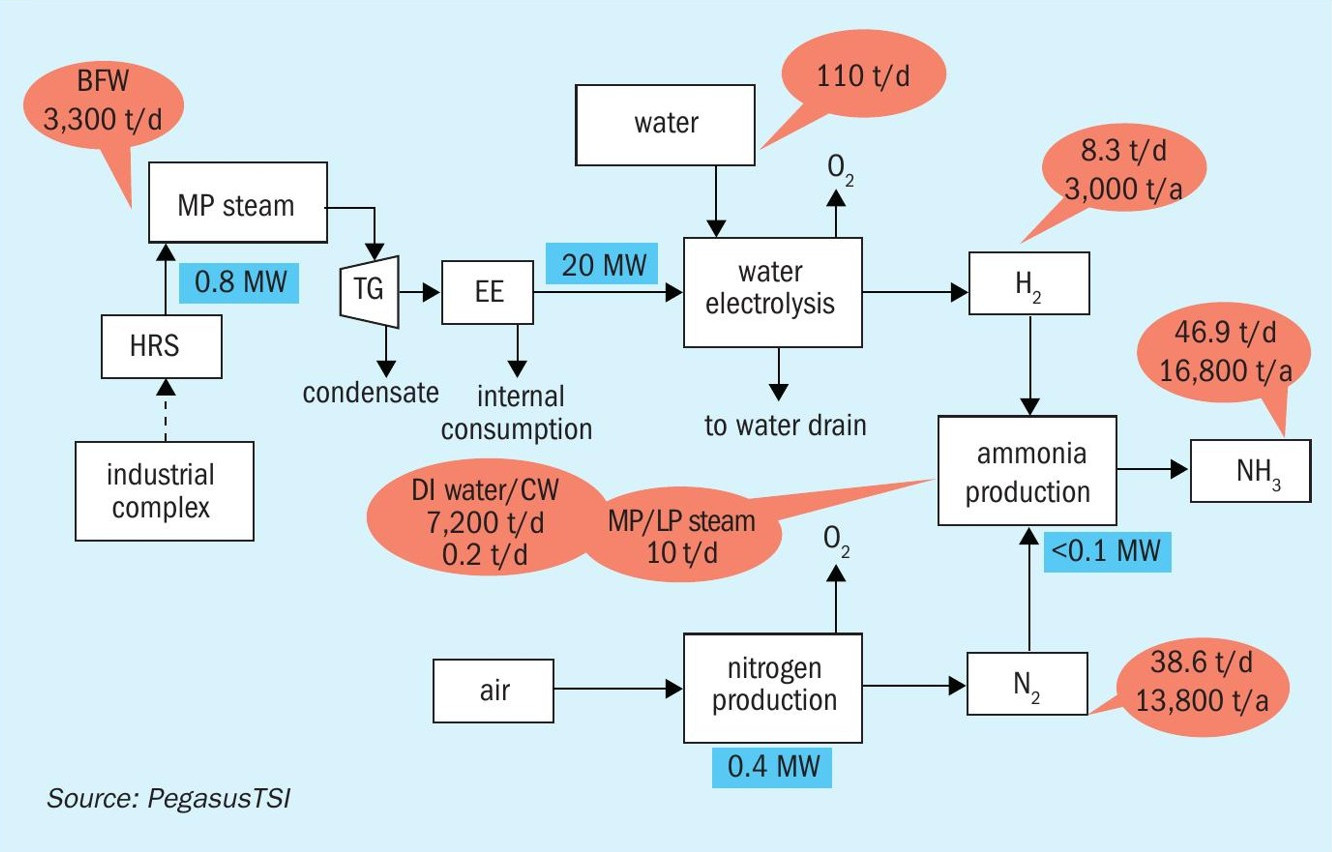
The green hydrogen can also be used in the production of green ammonia , which can be used in the production of GMAP fertilizer. The use of green ammonia in the production of GMAP fertilizer will have the impact of decreasing the CO2 footprint, due to replacement of the ammonia produced via the natural gas steam reforming route, which has a CO2 footprint of 2t CO2 /t ammonia.
In this case the 3,000 t/a of green hydrogen can be used for the production of 16,800 t/a of green ammonia (Fig. 8).
CO2 emissions and footprint
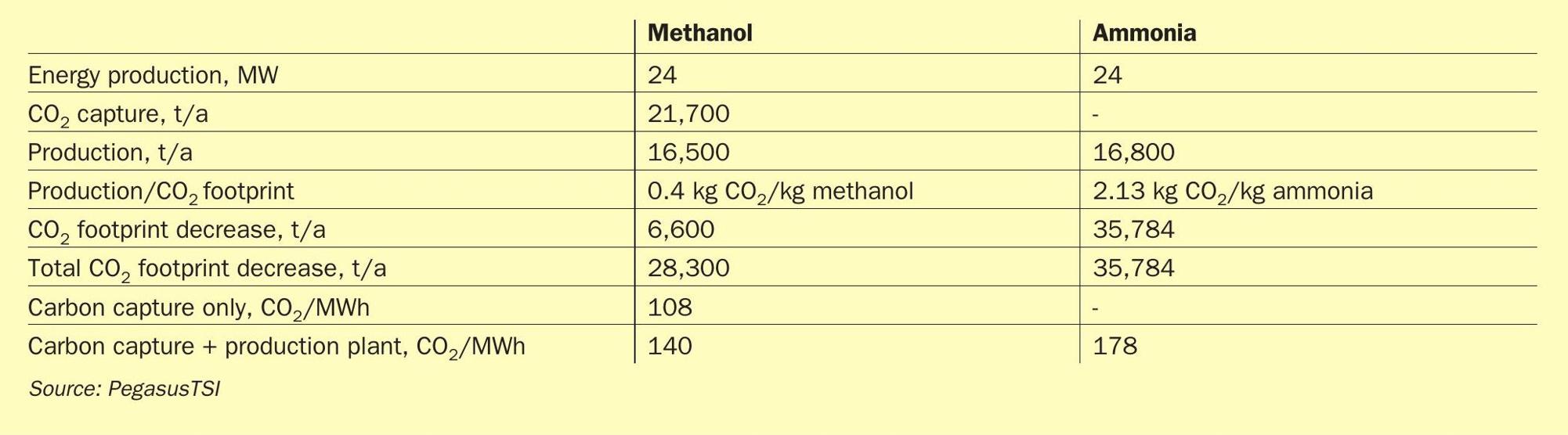
The CO2 emissions and footprint benefits of producing green methanol versus green ammonia in a fertilizer industrial complex has been studied.
Table 1 compares the impact on CO2 footprint between methanol and ammonia production.
If the 24 MW of clean energy from the sulphuric acid plant were used for the production of green methanol the reduction in CO2 footprint would be 28,300 t/a. Alternatively, if the clean energy were used for the production of green ammonia the reduction in CO2 footprint would be 35,784 t/a.
The production of green ammonia therefore has a bigger impact in decreasing the CO2 footprint in the fertilizer complex when using the same amount of green hydrogen.
Economics
Green methanol
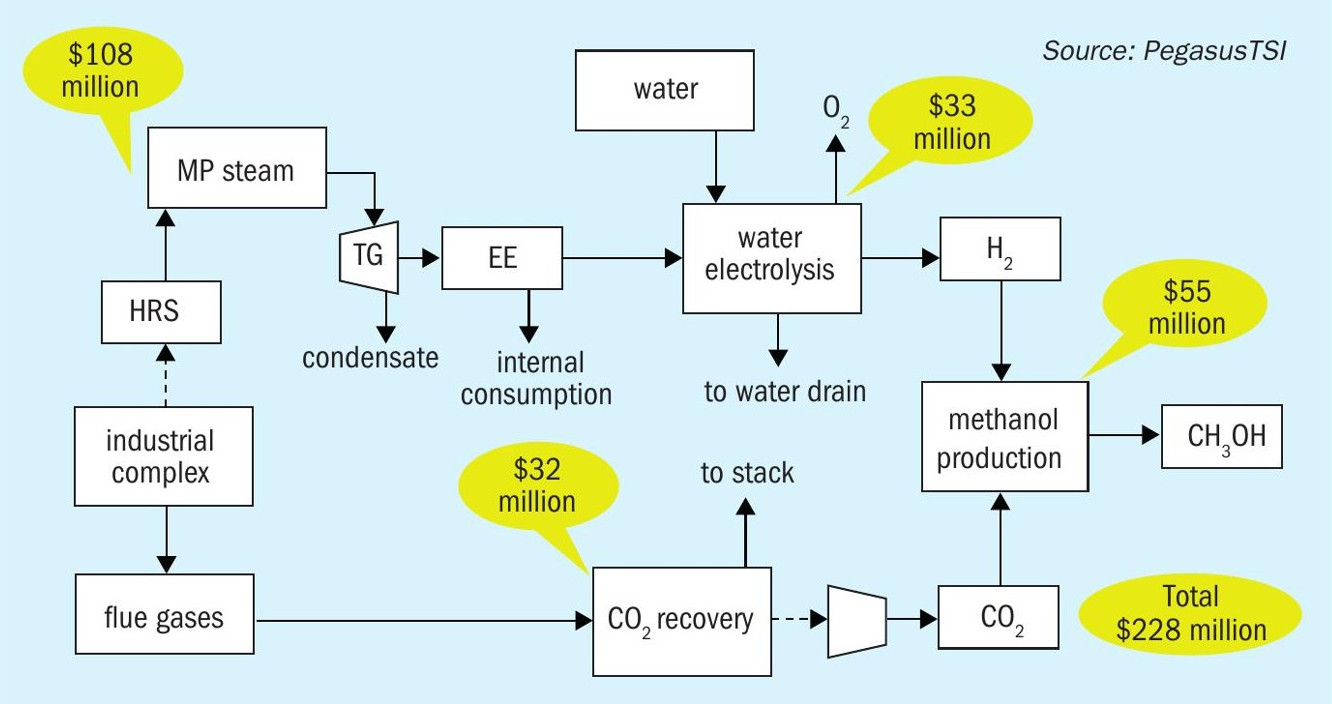
The total investment to produce 16,500 t/a of green methanol is estimated to be $228 million, which includes the two sulphuric acid plant heat recovery systems, steam turbine system, electrolyser, CO2 recovery system and the methanol plant (Fig. 7).
The economic feasibility of green methanol on this scale is based on the following parameters:
- Investment: $228 million
- Green methanol production: 16,500 t/a
- CO2 Capture: 21,700 t/a
- CO2 carbon credit: 50 $/t
- ROI: 8%
Under current market conditions (1Q 2022), the green methanol price is around 1,290 $/t, which exceeds the market price for methanol, which would be in the order of 500 $/t.
Despite the fact that the green methanol has a higher market price, there could be a demand for product that can decrease the CO2 footprint of other companies that use methanol as a raw material.
Green ammonia
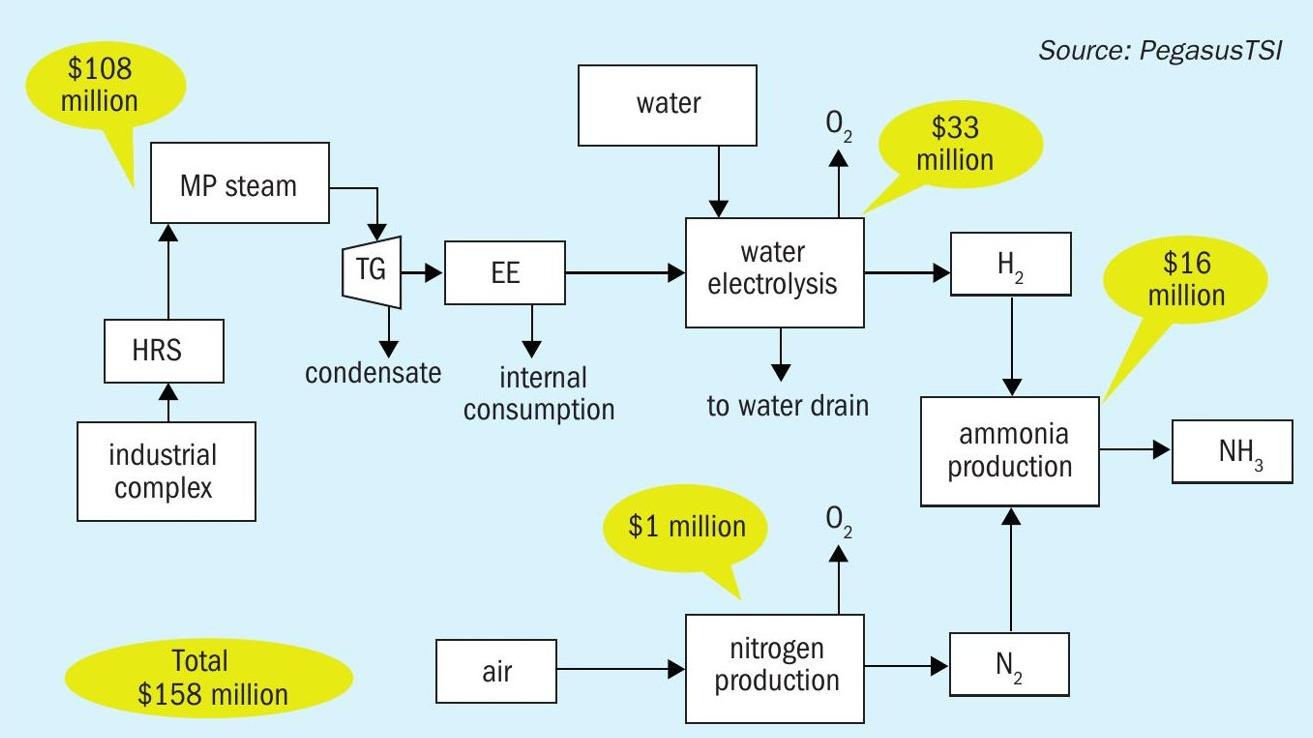
The total investment to produce 16,800 t/a of green ammonia is estimated to be $158 million, which includes the two sulphuric acid plant heat recovery systems, steamturbine systems, electrolyser, CO2 recovery system and the ammonia plant (Fig. 9).
The economic feasibility of green ammonia on this scale is based on the following parameters:
- Investment: $158 million
- Green ammonia production: 16,800 t/a
- CO2 footprint decrease: 35,784 t/a
- ROI: 8%
Based on current market conditions, the green ammonia price is 900 $/t, which exceeds the current market price for ammonia, which is in the order of $500 $/t.
Despite the fact that the production cost of green ammonia is higher than market prices, there could be demand for fertilizer products that have a lower CO2 footprint.
An additional consideration to improve the feasibility of green ammonia production is the potential for modification of an existing ammonia facility to use green hydrogen to decrease the capital investment and improve the feasibility of the project.
Conclusions
Technologies to decrease CO2 footprint are commercially available and the economic feasibility will depend on the ability of the market to pay a premium for green methanol or green ammonia.
A preliminary analysis comparing green methanol and green ammonia production shows that in an industrial fertilizer complex green ammonia production may be attractive when a high CO2 reduction is required with reduced capital investment.
A further factor that may be considered in a CO2 reduction strategy is the use of other sources of renewable energy, like wind or solar together with the clean energy coming from the heat recovery system in the sulphuric acid plant to increase the capacity and decrease the production cost.