Nitrogen+Syngas 391 Sep-Oct 2024

30 September 2024
A tailored approach to reducing GHG emissions
NITRIC ACID PRODUCTION
A tailored approach to reducing GHG emissions
Stefano Cicchinelli and Carmen Perez of Stamicarbon (MAIRE) explore the latest advancements in tertiary abatement technologies, their implementation in nitric acid plants, and the implications for the fertilizer industry.
The nitric acid production industry plays a crucial role in the global agricultural sector, primarily due to its use in producing nitrogen-based fertilizers. However, the environmental impact of nitric acid plants, particularly their greenhouse gas emissions, has become a significant concern. Tertiary abatement technology presents a viable solution to reduce these emissions and align with increasingly stringent environmental regulations.
Nitric acid production
Nitric acid production is one of the key processes of the fertilizer industry, essential for synthesising ammonium nitrate. Most nitric acid plants rely on the high-temperature catalytic oxidation of ammonia, a method known as the Ostwald process. This process runs in two stages: First, ammonia (NH3) is oxidised to produce nitric oxide (NO), which is then further oxidised to form nitrogen dioxide (NO2). Second, nitrogen dioxide (NO2) is absorbed in water (H2 O) to produce nitric acid (HNO3).
Stamicarbon, the nitrogen technology licensor of NEXTCHEM’s Sustainable Technology Solutions BU (MAIRE group) offers the NX Stami Nitrates™ technology, which utilises its proven mono- or dual-pressure design. Mono-pressure technology in nitric acid production naturally operates at a single pressure level, optimising ammonia oxidation and nitric acid absorption with a compact layout and in a cost-effective manner. In contrast, dual-pressure technology works with two different pressure levels – medium pressure for ammonia oxidation and high pressure for nitric acid absorption – allowing for higher production capacities and greater energy efficiency. This layout increases catalyst life and reduces operational costs, making it a preferred choice for high-capacity nitric acid plants.
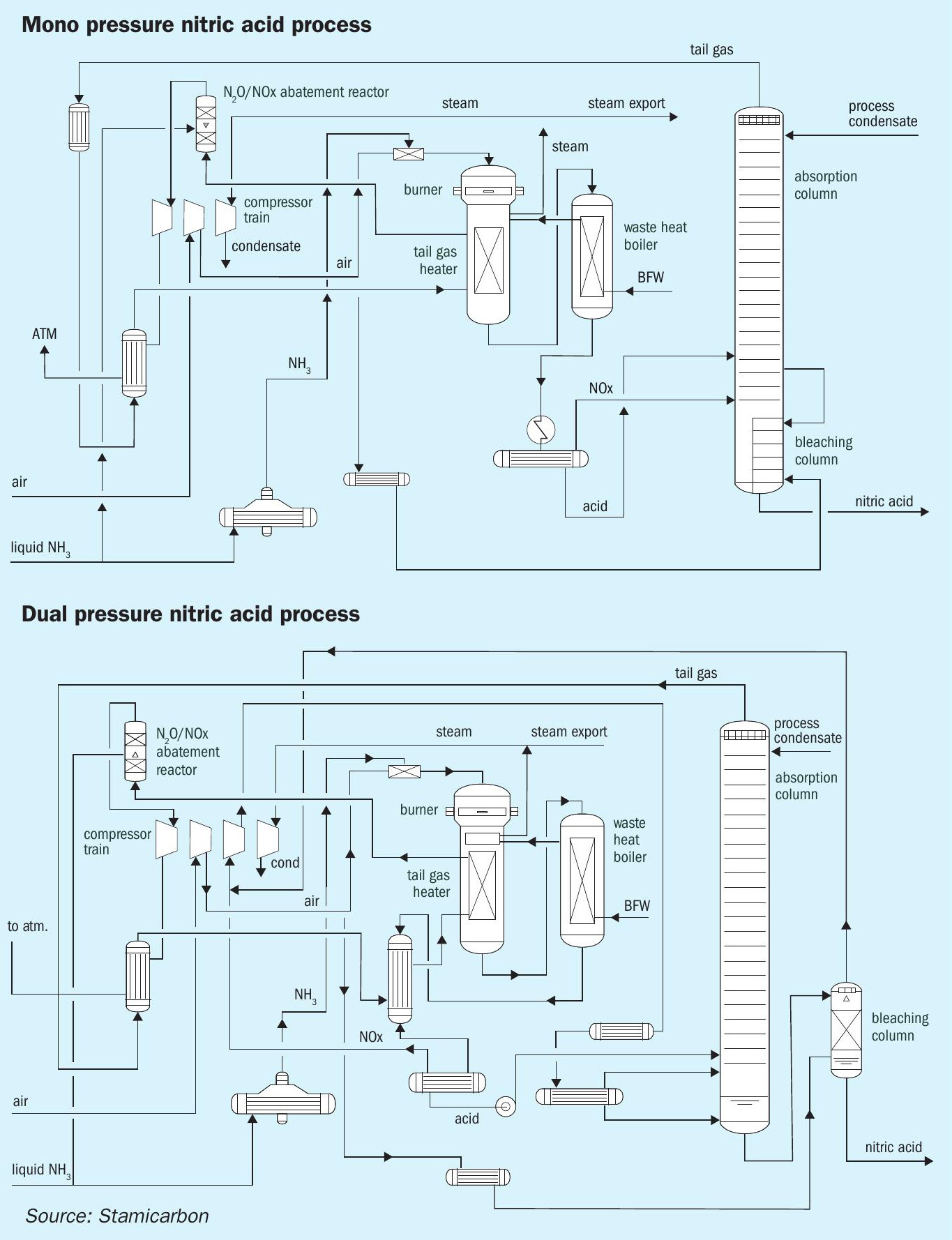
Renowned for their longevity and low maintenance requirements, Stamicarbon nitric acid plants excel in maximising high-quality steam export (see Table 1) and, hence, energy efficiency. Despite continuous improvements in the design of next-generation plants over the years, the performance of existing installations – some of which have been in service for 40 to 50 years – remains competitive in the market, making their dismantling for the construction of new plants unjustified.

Environmental impact
Notably, emissions were not a significant concern in plants built until the 1990s, when the legislation on NOx emissions began to be implemented. Regulations on N2O emissions were introduced later and are still not enforced in some countries. Over the years, there has been an increasing push to curtail emissions from nitric acid production facilities, largely driven by legislative mandates aimed at reducing greenhouse gas emissions and mitigating climate change. In certain situations, companies have also taken voluntary steps towards a greener transition, including the reduction of N2O emissions, as part of their corporate social responsibility and sustainability goals.
Although most existing nitric acid plants are operating smoothly, modernisation is necessary to enhance sustainability. For new nitric acid plants designed by Stamicarbon, a tertiary abatement reactor capable of effectively removing or nearly eliminating NOx and N2O from tail gases released into the atmosphere is offered as a standard solution. This reactor integrates seamlessly with the production technology where the tail gas temperature is around 480°C. For other existing plants with tail gas temperatures between 450 and 500°C, this tertiary abatement system can be implemented with minimal modifications to the plant layout, making it a viable option for upgrading older facilities to meet modern environmental standards. In this layout, N2O is removed by thermal decomposition. For plants where the tail gas temperatures ranges between 350 and 500°C, the same solution can be applied, but N2O should be removed by reduction with natural gas.
Solutions for low tail gas temperatures
While many nitric acid plants operate at high tail gas temperatures, challenges arise when plants run at low tail gas temperatures, significantly below 350°C. In these cases, tailored solutions are required to maintain effective abatement of NOx and N2O emissions without necessitating extensive modifications to downstream equipment, such as the need to upgrade the material of the expansion turbine. Stamicarbon is implementing new catalysts that can perform efficiently at these lower temperatures. In some cases, using the advanced catalysts can achieve competitive N2O emission values, thereby simplifying necessary layout modifications and reducing investment costs.
A current solution involves increasing the tail gas temperature because N2O abatement must occur at higher temperatures. This approach demands using external energy sources to heat the tail gas at the entrance of the abatement system and subsequently lowering the temperature to meet the maximum design value of the tail gas expander downstream of the abatement reactor. Modifications may involve adding an electric heater, as shown in Fig. 2, or a gas-fired furnace. However, this raises an important question: is it wise to reduce greenhouse gases from tail gases while directly or indirectly producing other greenhouse gases? This consideration is crucial as it underscores the balance between achieving immediate emission reductions and the broader goal of sustainable and environmentally responsible operations.
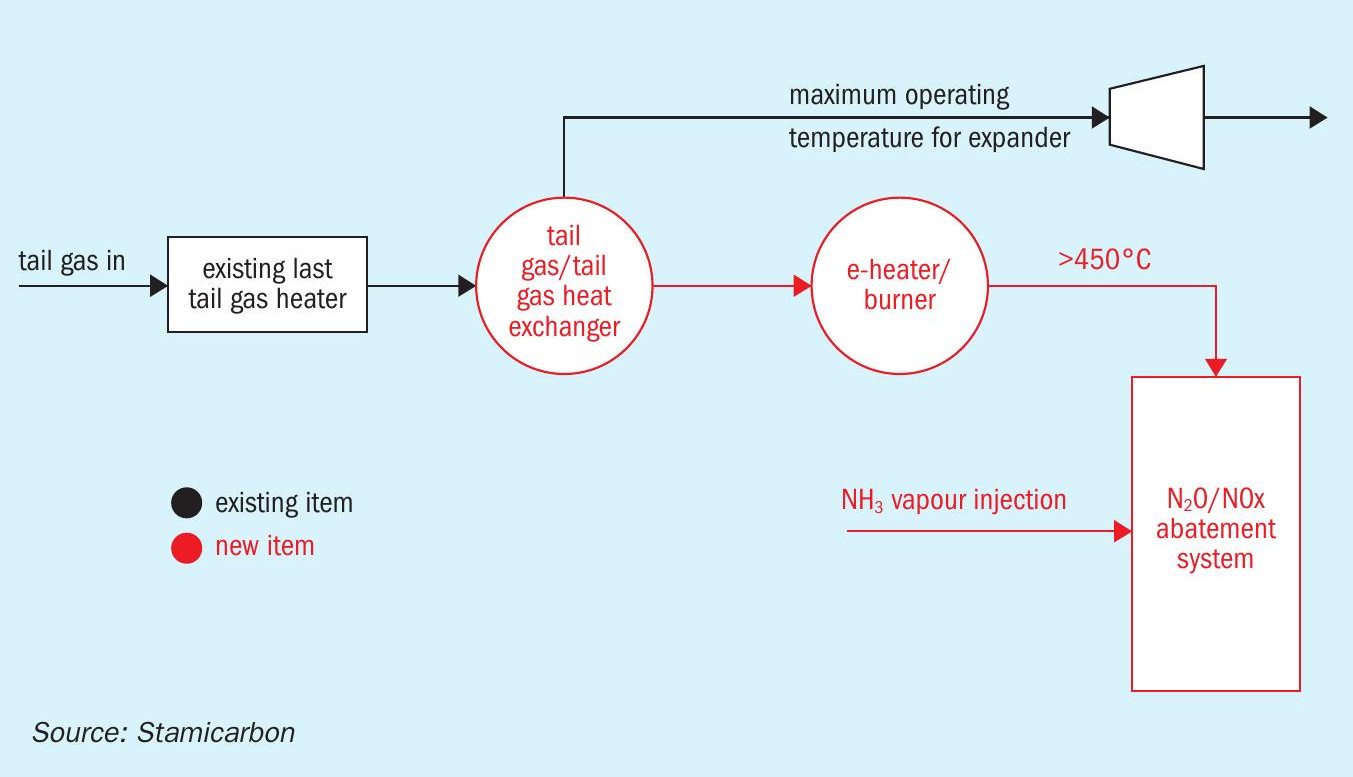
Choosing between tertiary and secondary catalysts
Another relevant consideration arises when plant owners must choose between adding a tertiary or secondary catalyst, as illustrated in Fig. 3. While adding a secondary catalyst to the existing NH3 burner might seem cost-effective and less impactful on the plant layout, careful analysis often reveals that the secondary catalyst is not the optimal solution.
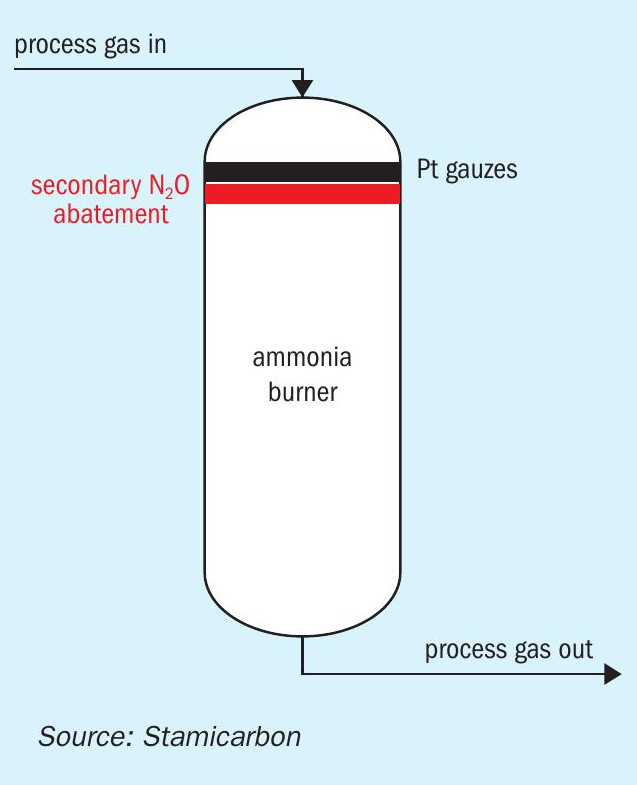
First, existing burner conditions must be assessed, as many of these units have been operational for many years, making substantial modifications challenging. Therefore, the mechanical integrity of the equipment and the potential pressure drop in the process gas must be evaluated. Second, reactor modifications and catalyst installation entail several days of downtime, resulting in production losses. Lastly, it is not possible to achieve high conversion rate of N2O with secondary abatement since the reactor size has a size limitation and it is expected that more stringent regulations will be issued in the near future resulting in it not being possible to keep adding catalyst to the secondary catalyst basket.
A more reasonable and future-proof approach might involve replacing the entire burner with a new one specifically designed to accommodate the secondary catalyst. However, the economic feasibility of this solution depends on whether the plant is prepared to simultaneously increase its capacity to optimise the return on investment. Beyond the mere reduction of N2O emissions from tail gases, the purpose extends far deeper, a topic that will likely feature in an separate article.
Stamicarbon is an advocate of tertiary abatement as the superior alternative. This equipment can be installed while the plant is operational, requiring minimal shutdown time for line connections. Additionally, the greater N2O reduction leads to significant carbon tax relief, making it a cost-effective and sustainable choice in the long run.
Considerations for higher tail gas temperatures
Unlike plants operating at low tail gas temperatures, certain nitric acid plants employ technologies where the tail gas must reach very high temperatures upstream of the expansion turbine, typically between 700-800°C. In these plants, N2O removal is generally not in place. Instead, a non-selective catalytic reduction (NSCR) reactor is used to increase the temperature and reduce NOx emissions simultaneously.
However, these installations already feature NOx abatement reactors that utilise expensive NSCR catalysts. An alternative approach proposed by Stamicarbon is to consider replacing the old catalyst, as shown in Fig. 4, with a new one, such as the honeycomb catalyst based on precious metal. This replacement minimises plant modifications while maintaining the overall energy balance. However, it is important to evaluate the required volume of catalyst and the price of these precious metals compared to the additional operational cost of having an electrical heater or a gas fired burner.
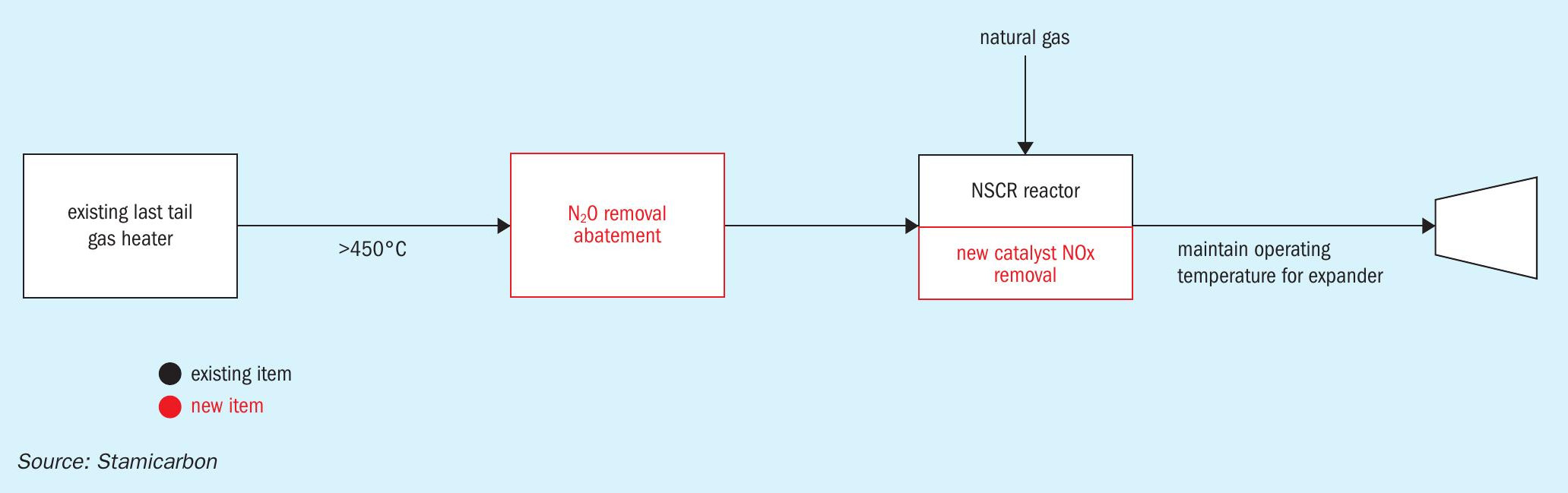
As we navigate the complexities of temperature requirements, catalyst choices, and energy balances, the path forward becomes clearer. Whether seamlessly integrating with existing technologies or exploring innovative catalysts, Stamicarbon is committed to finding optimal solutions for minimising the fertilizer industry’s environmental impact.
Conclusions
In the context of nitric acid plants, the pursuit of sustainability and environmental responsibility is vital. While existing installations have demonstrated technological reliability over decades, the need to reduce emissions remains crucial both for addressing greenhouse gas pollution and complying with legislative requirements.
By embracing tertiary abatement, emissions are addressed and pave the way for a sustainable future where longevity, performance, and environmental stewardship coexist.





