Nitrogen+Syngas 391 Sep-Oct 2024

30 September 2024
Decarbonisation of the maritime industry
EMISSIONS CONTROL
Decarbonisation of the maritime industry
As the industry pushes towards more sustainable practices, ammonia is emerging as a promising alternative fuel for ships. Effective management of by-product NOx, NH3 and N2O emissions from the combustion of ammonia is crucial to the success of ammonia as an alternative fuel. A new catalyst has been developed by Enercat to treat these three molecules in one bed. Jean-Rémi Stephany and Emmanuel Rohart of Enercat – Alsys Group report on this new technology which has been developed in marine ammonia combustion engine conditions.
The maritime industry, a cornerstone of global trade, is currently facing increasing pressure to reduce its environmental footprint. Traditional marine fuels, primarily diesel, produce significant greenhouse gas and contribute extensively to air pollution. As the industry pushes towards more sustainable practices, ammonia is emerging as a promising alternative fuel for ships.
In many cases, ammonia demonstrates highly interesting benefits for the marine transport transition. Its energy density in volume of 13.6 GJ/m3 , ranks it between hydrogen and gasoline. In addition, its favourable storage properties allow it to be stored as a liquid at relatively moderate pressures and temperatures. Moreover, ammonia benefits from existing production and transportation infrastructure that can be easily adapted for maritime applications. Finally, recent advancements in engine technologies are making it feasible to retrofit existing diesel engines to run on ammonia, further easing the transition.
Beyond all the previously listed advantages of ammonia as a fuel, one of the most compelling reasons to consider ammonia as a marine fuel is its potential for zero carbon dioxide (CO2) emissions, which makes it a very attractive option for meeting the International Maritime Organization’s (IMO) ambitious targets of halving greenhouse gas emissions in shipping by 2050.
However, while ammonia combustion releases no CO2 in the atmosphere, careful attention must be paid to the possible formation of by-products during the process such as nitrogen oxides (NOx), ammonia (NH3) and nitrous oxides (N2O). The first two molecules are well known for their impact on human health, while the third one is a potent greenhouse gas with a global warming potential that exceeds that of CO2 by 300 times.
To make ammonia not only a promising but a serious candidate for energy transition in the marine industry, effective management of these emissions is crucial.
Simultaneous treatment of NOx, NH3 and N2O
On the one hand, selective catalytic reduction (SCR) systems are well known solutions to mitigate NOx emissions with NH3 injection in diesel engines post-treatment. On the other hand, some catalytic formulations have proved their ability to treat N2O in various chemical processes such as nitric acid production plants.
Enercat has developed and patented a catalytic formulation used for more than 15 years for the simultaneous treatment of NOx and N2O with NH3 injection in nitric acid plants.
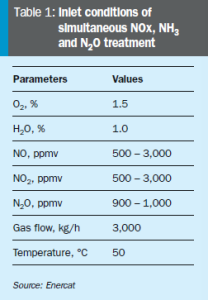
Based on this technology, Enercat has developed and supplied in 2022 a tailor-made flue gas treatment solution for a chemical plant in France. The system was designed to reduce large quantities of NOx, with very high and sudden variations, while abating N2O in the same reactor (inlet conditions shown in Table 1). The specificity of the process was the variability of the NOx concentration at the inlet, driving Enercat to inject NH3 in excess to achieve the required NOx conversion. The drawback of injecting large quantities of NH3 was the potential NH3 slip at the stack when the NOx to be treated at the inlet suddenly dropped.
Therefore, the solution relied on a single reactor process containing two catalytic beds in series (see Fig. 1). The first bed allows N2O and NOx to be treated simultaneously with NH3 injection using Enercat’s patented catalytic technology. The second bed reduces potential ammonia emissions using Enercat’s “ammonia slip” catalyst (ASC). The combination of the two beds allows the conversion of more than 99% NOx and 95% N2O without releasing a single ppm of NH3 at the stack.

From chemical plant to marine applications
Based on the internal developments described previously, Enercat decided to push innovation further and develop a catalyst capable of treating the three molecules in one bed. This technology has been developed in marine ammonia combustion engine conditions, as the need for treating NOx, NH3 and N2O is a key for the success of ammonia as an alternative fuel.
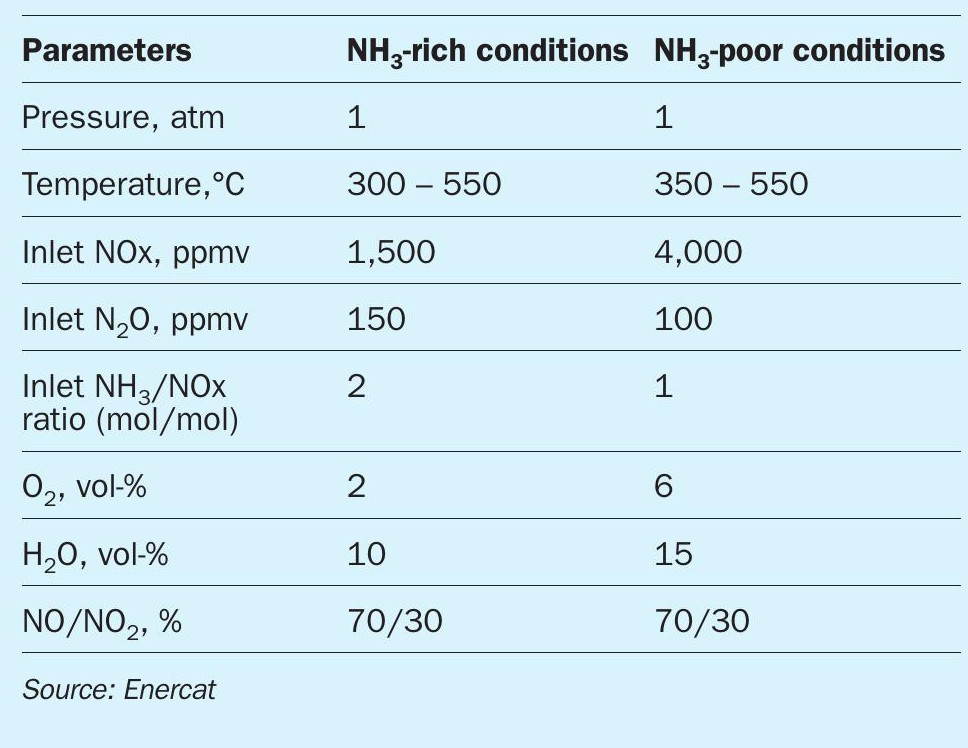
Two types of conditions were shortlisted (see Table 2). One with a large quantity of residual unburned NH3 . And the second with the stoichiometric quantity of ammonia required to ensure NOx reduction over the catalyst. The developed technology was compared to a classic DeNOx SCR technology used in diesel engines post-treatment.
The first results show that the performance of the classic SCR catalyst is high for the reduction of NOx below 400°C, but the catalytic formulation shows no activity for N2O decomposition. Furthermore, above 450°C, an important decrease of DeNOx performance is observed, associated with rocketing N2O formation on the catalyst.
On the other hand, the catalyst developed by Enercat shows very good performances at 450°C and above in both conditions, especially compared to the classic SCR DeNOx catalyst, allowing full conversion of the three targeted molecules (NOx, NH3 and N2O) with only one catalyst. Although the current catalytic formulation enables very high conversion rates to be reached, ongoing further developments should enable the performance to be increased at lower temperatures.


This very promising solution allows the implementation of compact and cost-effective post-treatment systems for ammonia combustion engines, making ammonia as a fuel an even more serious candidate for the decarbonisation of the maritime industry.