Sulphur 389 Jul-Aug 2020
31 July 2020
Caustic scrubbing of molten sulphur vent streams
MOLTEN SULPHUR STORAGE AND TRANSPORT
Caustic scrubbing of molten sulphur vent streams
With increasing frequency, companies that have molten sulphur on site must put environmental controls on the vent streams from molten sulphur pits, storage tanks and loading operations. This article* describes the typical characteristics of molten sulphur vent gas streams as well as some of the important chemistry related to these systems in caustic scrubbers. Solids deposition issues observed in the field with caustic scrubbers operating on actual molten sulphur vent gas streams are presented. Design and operational strategies to mitigate plugging in molten sulphur vent gas scrubbers are also summarised in this article by D. J. Sachde, K. E. McIntush, D. L. Mamrosh, and C. M. Beitler of Trimeric Corporation.
The removal of hydrogen sulphide (H2 S) from sour gas streams can be achieved with many different technologies, one of which is caustic scrubbing. Caustic solution is often used in various processing steps in refineries and its availability and familiarity make it a reasonable choice for removing H2 S from vent streams from molten sulphur operations. While caustic scrubbing is an established technology that can readily remove H2 S, its use on molten sulphur vent streams is complicated by the presence of elemental sulphur that can plug different areas of the processing equipment.
The elemental sulphur in the vent gas and in the associated caustic scrubber system may be in various forms (vapour, aerosols/fog, sulphur sols [in liquid], and solid particulate) so that solids plugging can occur in the piping from the source and in the scrubber itself. Minimising sulphur plugging can be accomplished by including certain design features in the equipment and by managing the dissolution of elemental sulphur in the caustic solution.
Molten sulphur vent gas
Vent streams from molten sulphur pits, storage tanks, and loading operations are generally composed primarily of air or nitrogen, but also contain H2 S, sulphur dioxide (SO2 ) and elemental sulphur (S8 ) that must be treated to meet regulatory requirements and prevent unwanted solids deposition. The amount of sulphur compounds in the vent gas varies significantly depending on the source of the molten sulphur and the handling conditions. For example, the sulphur produced in the Claus process in an oil refinery contains soluble H2 S and hydrogen polysulphides (H2 Sx ). During the storage of the sulphur, the H2 Sx compounds will decompose slowly to elemental sulphur and H2 S as the sulphur cools and is agitated.
The dissolved H2 S in the liquid sulphur can desorb into the gas phase. With a stagnant head space, the H2 S then accumulates in the vapour space above the liquid sulphur. Sweep gas is often used to keep the H2 S concentration above the surface of the molten sulphur below a maximum of 25% of the lower explosive limit for H2 S in air1 . However, the health and safety hazards of H2 S and SO2 along with environmental regulations may necessitate treatment of any vent vapour streams from the sulphur handling operations.
The presence of elemental sulphur represents a unique challenge when treating vent gas in molten sulphur operations. The vent gas is commonly assumed (conservatively) to be saturated at its temperature and pressure with elemental sulphur vapour. Literature sources indicate that elemental sulphur vapour may be present as S2 , S4 , S6 , and S8 with larger molecules (through S12 ) found in certain cases and predicted by theory2,3 . At the conditions expected in sulphur vent gas (250-300°F/121-149°C), the larger molecules, in particular S8 , are favoured2 .
The problem is further complicated by the various physical phases and forms of elemental sulphur that may be present in the vent gas and associated caustic scrubber system, including:
- sulphur vapour;
- sulphur aerosols/fog/mist or entrained sulphur droplets;
- sulphur sols;
- sulphur particulates/solid.
The sulphur vapour can change phases as it leaves the high temperature molten sulphur operation and enters the lower temperature scrubbing system, and may be in any of the forms listed above. Thus, the actual amount of elemental sulphur travelling through the vent system/caustic scrubber system is difficult to quantify. Further, H2 S in the gas can also react with SO2 and/or oxygen from the sweep air to form additional elemental sulphur.
*This article on reliable design and operation of caustic scrubbers for molten sulphur storage and transport vent streams by Darshan Sachde, Kenneth E. McIntush, Darryl L. Mamrosh and Carrie Ann M. Beitler of Trimeric Corporation is a modified version of a paper previously presented at the Brimstone Sulfur Symposium. 12
Industry experience
Although the exact number of caustic scrubbers that treat vent gas from molten sulphur systems is not known, a knowledgeable industry contact from a company that stores, markets, ships, and uses molten sulphur (“merchant sulphur industry” for short) guessed that there might be on the order of 50 caustic scrubbers in this service around the world. Further, Trimeric’s exposure to some data from operators of oil refineries and gas plants suggests that there are at least a dozen or more in oil refineries, probably mostly in the United States.
As a result of work with clients with these systems, Trimeric gathered confidential input from operating companies (primarily refining) regarding caustic scrubbers in this application. As detailed later, features mentioned that were associated with improved operation included: the use of venturi contactors prior to conventional packed scrubbers, caustic sprays at the point where the vapour line enters the scrubber, and on-line washing of the scrubber overhead equipment and lines to clean out those areas.
Important chemistry
Because H2 S and SO2 are hazardous gases with strong odours, emissions tend to be limited by regulations, and the vent streams from the molten sulphur handling operations commonly must be treated to remove these compounds. Caustic scrubbing of vent streams usually involves counter-current contacting of the gas phase with a recirculating caustic solution in a packed or tray tower. The chemistry and operation of the caustic scrubber for H2 S and SO2 removal is not the focus of this article and is not covered in detail. However, it should be noted that reliable design of the caustic scrubber in this application requires a full understanding of the chemistry to prevent salts precipitation, meet treatment specs, and maximise utilisation of the caustic solution4 .
Elemental sulphur dissolution and caustic chemistry
This section will provide a brief overview of the dissolution of elemental sulphur in aqueous caustic solutions, with a focus on data available in the literature. It is important to note that the following discussion is limited in scope in several ways compared to the complexity present in a caustic scrubbing system applied to a molten vent gas stream in the field:
- Other species present in vent gas streams (e.g., H2 S, SO2 , and oxygen) will potentially complicate the chemistry significantly.
- Reaction pathways will change with specific operating conditions, particularly pH and temperature. Literature data may not be representative of specific conditions in a caustic scrubber.
- The experimental methods used to measure dissolution may not be representative of a caustic scrubber (particle size distribution of elemental sulphur, agitation, temperature control, etc.).
Even sulphur dissolution in neat caustic solution includes multiple mechanisms that impact the rate and extent of dissolution:
- wetting of solid sulphur particles by aqueous solution;
- physical solubility of elemental sulphur in the aqueous solution;
- chemical reaction of caustic solution with elemental sulphur;
- diffusion of reactants and products through the solid/liquid interface boundary layer.
These mechanisms, in turn, are a function of the physical properties and conditions (temperature, pH, concentrations, etc.) of the solution. In general, sulphur has a low physical solubility in caustic solution, but chemical reactions enhance solubility. The most stable form of elemental sulphur in the temperature range of interest is S8 (orthorhombic sulphur), which is hydrophobic and not readily wetted by caustic solution. Some research indicates that reaction rates are strongly influenced by the surface area of elemental sulphur particles (i.e., reactions take place on the surface of the particles)5, 6 . Therefore, wetting of elemental sulphur must be considered alongside any reactions. This article is focused on total solubility of elemental sulphur in caustic solutions (combination of physical solubility and chemical reaction), but in practice the other mechanisms discussed will be important.
The reactions of elemental sulphur with caustic can include multiple pathways depending on the conditions of the solution. However, two general routes for the reaction of elemental sulphur with caustic solution will be considered:
- disproportionation (also called alkaline dissolution of elemental sulphur);
- addition reaction with sulphide/bisulphide.
Disproportionation reaction
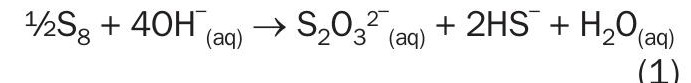
(Simplified chemistry and stoichiometry represented in reference 16)
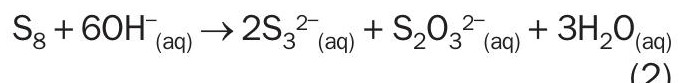
(Alternative disproportionation chemistry proposed in reference 15 that reflects formation of polysulphides)
Addition reaction/poly sulphide formation
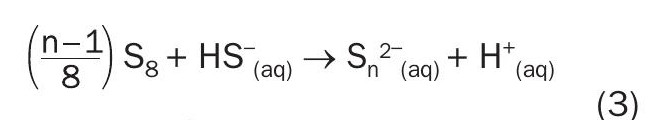
(Presented in reference 14)
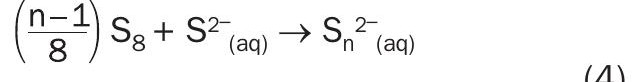
(Generic form of reaction presented in reference 15, where n=2)
The disproportionation reaction is written in two forms that are presented in literature (reactions (1) and (2)). Reaction (2) yields a polysulphide (S3 ) species. The chemistry of polysulphides is beyond the scope of this paper; however, polysulphides are generally thermodynamically unstable and will decompose in the presence of caustic7 . Therefore, only reaction (1) will be considered further in this article.
This article assumes that the sulphur dissolves via reactions (1) and (3) to calculate a stoichiometric limit in the following table as follows:
- Every 4 moles of caustic present (either NaOH or KOH) consumes ½ mole of elemental sulphur (S8 ) and produces 2 moles of bisulphide (HS– ) according to equation (1).
- The two moles of bisulphide can then consume ½ mole of elemental sulphur to produce polysufide in the form of S3 2– according to equation (3).
- In total, 1 mole of elemental sulphur is consumed for every 4 moles of caustic consumed for this proposed pathway.
- The stoichiometric limit of solubility can then be calculated based on the total caustic present in solution (weight % of caustic).
The choice between addition reactions (3) and (4) is unimportant to the calculation of the stoichiometric limit – bisulphide or sulphide will consume the same stoichiometric amount of elemental sulphur.
Note that this approach is only approximate – a specific reaction pathway was selected and limitations on physical solubility/reaction rates are ignored in the calculation. However, Table 1 indicates the estimated stoichiometric limit agrees reasonably well with the experimental data. Table 1 includes solubility data of elemental sulphur in caustic solutions from various literature sources8 , 9, 10 . The first set of data (shaded in grey) comes from reference 8, the second set of data (shaded yellow) comes from reference 9 and the last set of data comes from reference 10. The data point from the Sulphur Data Book10 lacks sufficient detail to be considered with the overall NaOH set and is reported only for general reference and completeness.
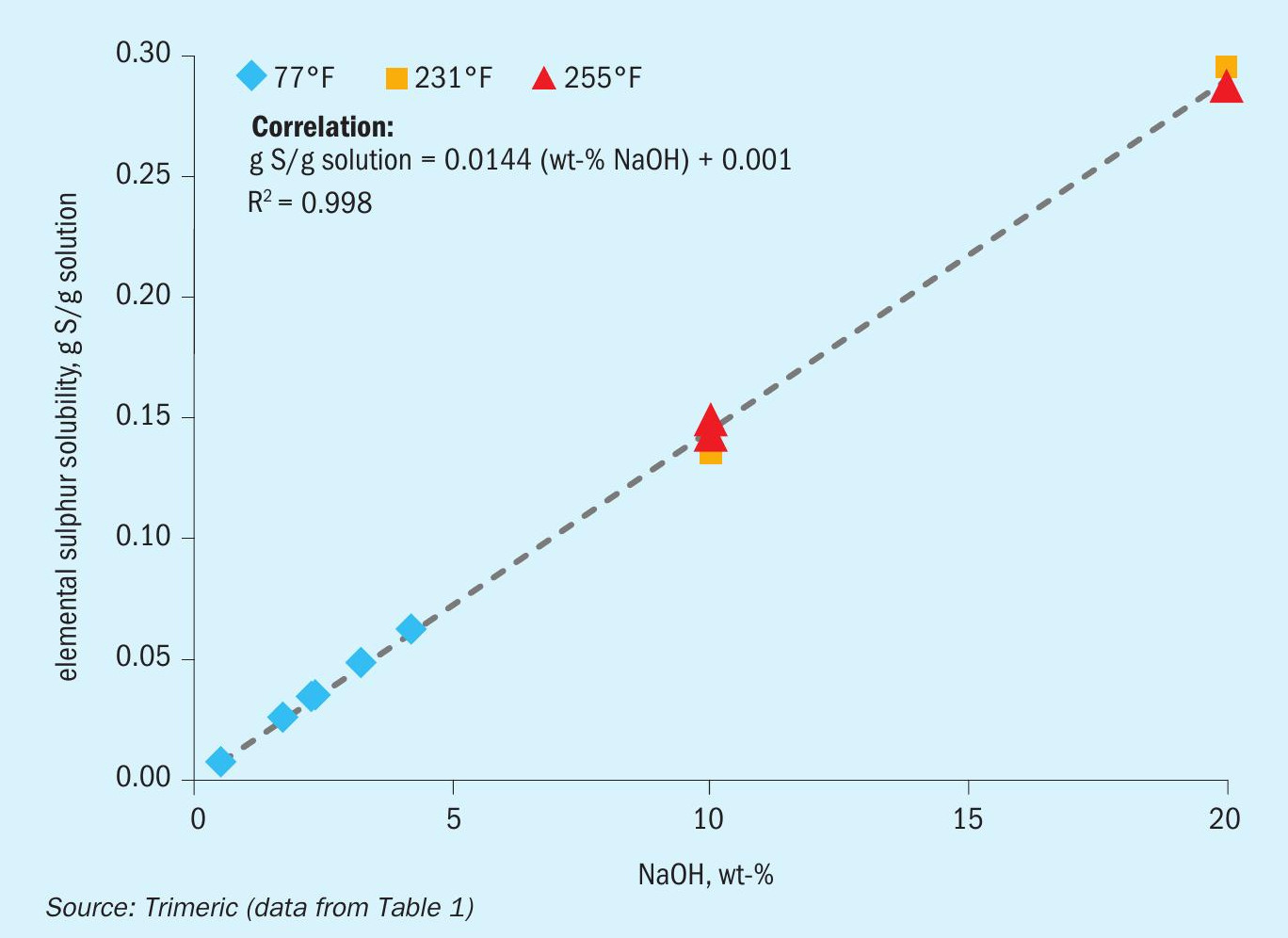
Fig. 1 plots the sulphur solubility as a function of caustic concentration in the relevant range of caustic scrubbers (< 20 wt-% NaOH).
The available data show little temperature dependence, as the sulphur solubility is effectively linear in caustic concentration despite the range of temperatures considered (77-255°F /25-124°C). These data indicate that with sufficient NaOH the elemental sulphur dissolution reactions go essentially to completion; the rate of sulphur dissolution is likely to be strongly influenced by temperature, however. Additional data over a broader range of temperatures and concentrations would be needed to confirm these findings. Similar data from literature is also available for KOH (not presented here)11, 12 . Recently, NH3 in water has also been shown to dissolve S33 .
Experimental and field data also support solubility of sulphur in more complex caustic solutions. For example, caustic solutions with sulphide species present (e.g., from the absorption of H2 S) have exhibited higher rates of dissolution and capacity for sulphur uptake than comparable neat sodium hydroxide solutions13, 14, 15, 16, 17 . In addition, DuPont Clean Technologies reported higher than expected caustic and hydrogen peroxide consumption (based on stoichiometry of H2 S and SO2 removal) in their scrubbing system when applied to a sulphur melter offgas18 . The higher consumption was attributed, in part, to disproportionation reactions of elemental sulphur in the scrubbing solution.
Difficulties with caustic scrubbers
The operability of caustic scrubbers in molten sulphur vent service can sometimes be difficult due to the formation of solids and plugging in the system. In some systems, the caustic scrubber can run for extended periods of time, while solids build-up in other systems requires frequent shutdown and cleanout of the equipment. The solids deposition can result from a variety of sources, as discussed below.
Salts precipitation
Solids can form if the operating temperature drops below the precipitation point for the salt species in solution. Localised cold spots in equipment, or instrumentation that is not insulated or heat traced properly can also be a source for salts precipitation. Liquid carryover with the gas from the scrubber system may lead to solid salts build-up in downstream equipment as well.
The formation of solids from salts precipitation can generally be avoided or minimised by understanding the solution properties at the operating conditions of the system. There are much literature data and some simulation tools that can be used to predict the precipitation temperature for the various components in solution. Once the precipitation temperature is known, the scrubbing system only needs to be operated at temperatures safely above this (10-15°F higher). If the necessary operating temperatures cannot be maintained in the system for some reason, a more dilute caustic solution could be used instead.
Elemental sulphur deposits
The more common and difficult to manage source of solids deposition is from elemental sulphur. Vent gas from molten sulphur operations contains sulphur vapour that can deposit on solid surfaces when the gas is cooled. The temperature of the vent stream entering the scrubber is often below the melting point of sulphur. Also, the temperature of the caustic scrubbing system itself is often low enough that any elemental sulphur vapour entering the scrubber changes phases.
Specifically, submicron aerosol or fog formation is a risk, as documented in condensers of Claus processes19 . Aerosols form when the sulphur vapour is rapidly cooled below the condensation point of elemental sulphur. Rapid cooling leads to a supersaturated vapour. If nuclei are present in the vapour and the kinetics of nucleation are sufficiently fast relative to the residence time of the supersaturated vapour, sulphur vapour will condense in the gas phase rather than forming a continuous liquid phase as in typical surface condensation processes.
Researchers have developed theoretical approaches to identify the limiting conditions (partial pressure of sulphur, temperature gradient, etc.) necessary for aerosol formation19 . Submicron aerosol particles are difficult to capture by traditional impaction methods (e.g., mist eliminators) as they will follow streamlines in the vapour.
Elemental sulphur in the liquid phase can exist as sulphur sols20 . Sulphur sols are emulsions of small droplets of liquid sulphur within a continuous bulk liquid phase in which the sulphur is sparingly soluble (e.g., aqueous solution). Sulphur sols can exist at temperatures far below the normal freezing point for elemental sulphur.
Stretford plants have experienced absorber plugging, “sticky” sulphur, and sulphur deposits in piping and downstream equipment that can be attributed to sulphur sols21 . In the Stretford process, a mechanism for sol formation exists because elemental sulphur is formed in the aqueous solution via oxidation of absorbed H2 S16 . However, experimental methods have also generated sulphur sols by directly contacting sulphur vapour with cold aqueous solution, which could be a mechanism for sol formation in a caustic scrubber20 .
Additionally, solid sulphur particulate is common in these vent streams. Solid sulphur can form by continued cooling of sulphur liquid in various forms (condensed vapour, sols, aerosols) in the system or be carried in the vent as solid particulate from the upstream process. Elemental sulphur is hydrophobic and will often build a layer of floating sulphur powder in the sump of the scrubber.
The location of sulphur deposits in the system coupled with knowledge of the different forms of sulphur may provide insight into the source of elemental sulphur in the system. For example, collection/plugging in mist eliminators indicates particles captured by impaction (larger aerosols or solid particulate). Sulphur that passes downstream of the scrubber (and mist eliminator, if present) in the vent gas may be indicative of small aerosols forming in the bulk vapour.
Elemental sulphur collecting or plugging in the lower portion of the column/sump or in liquid piping may be indicative of sulphur sols or captured sulphur which is not sufficiently reactive with the solution.
Design strategies to mitigate plugging of molten sulphur vent gas scrubbers
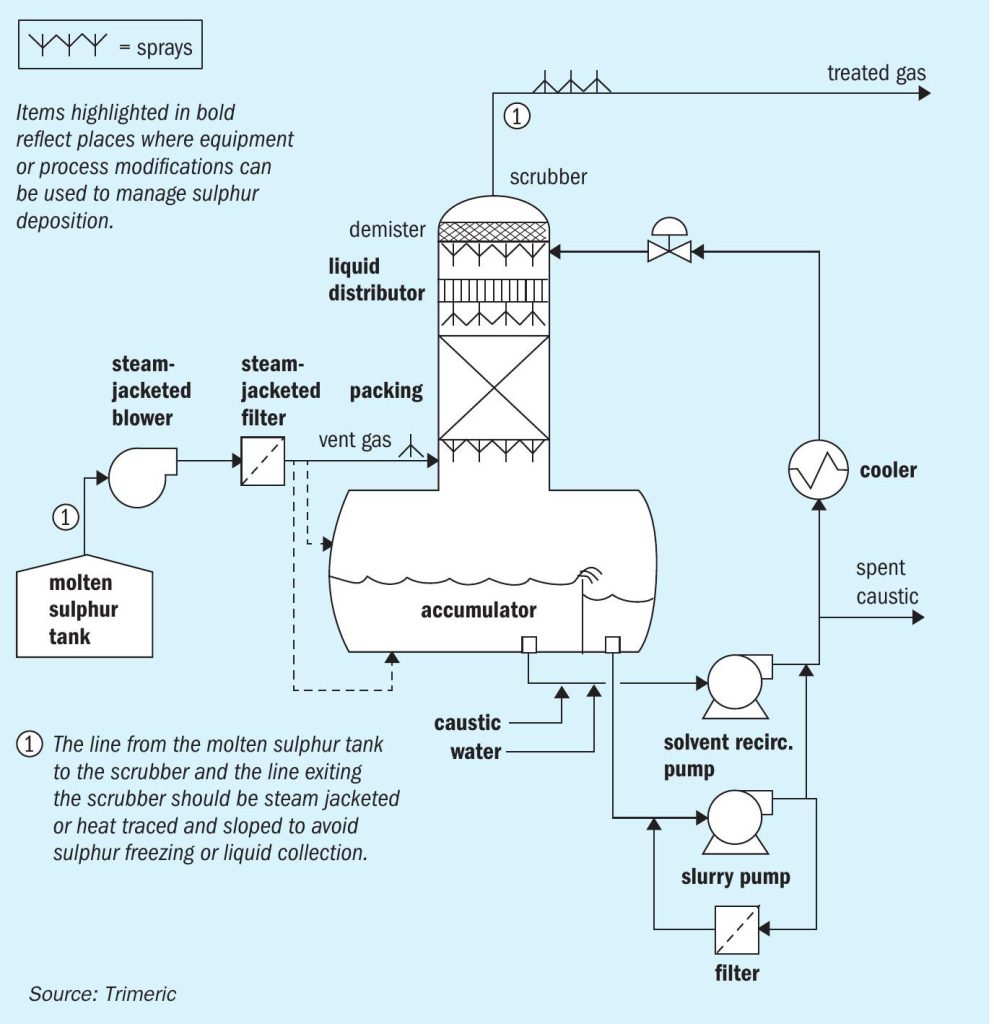
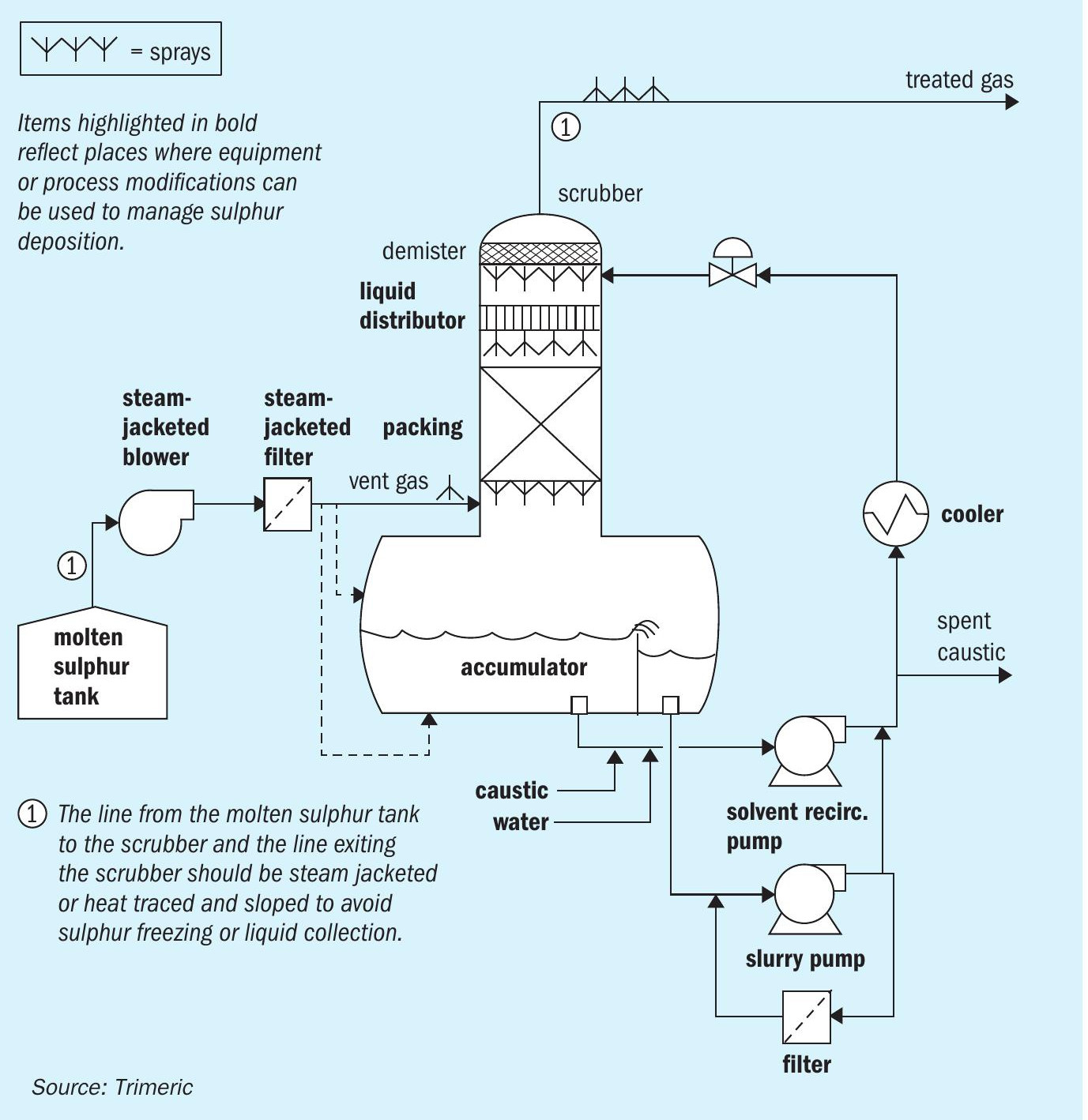
Caustic scrubbing is typically done using a variety of equipment including packed or trayed towers, sparged tanks, inline contactors, venturi contactors or a combination. An example of a common (not necessarily optimal) design for a packed tower caustic scrubber on a molten sulphur vent gas stream is shown in Fig. 2 as a reference for the reader through the remainder of this discussion.
In Fig. 2, the vent gas leaves the upstream process and is sent to the caustic scrubbing system via a motive device (blower, ejectors, eductors, etc.). The line from the molten sulphur tank to the scrubber and line exiting the scrubber should be steam jacketed or heat traced and sloped to avoid sulphur freezing or liquid collection.
The vent gas may pass through a steam jacketed filter as the first place where solid sulphur could be removed. The vent gas enters the caustic scrubber below the packed bed, where it countercurrently contacts caustic solution. The gas leaves the packed section of the column and passes through a demister which removes entrained liquid or large particulate. The treated gas leaves the column (typically vented).
The caustic solution absorbs the various sulphur species, and passes to the sump of the vessel. The solution is then pumped back to the top of the column, with a portion bled to remove spent caustic. A liquid filter may be used to remove any solids carried in the solution. The solution passes through a cooler (or heater in some cases), which controls the temperature of the caustic feed, and finally, the solution enters the top of the packed bed via a liquid distributor. Make-up caustic and water are added to the recirculating caustic stream.
There are many design features that can be incorporated into the caustic scrubber system to mitigate the plugging of the equipment from elemental sulphur solids as described in the subsections below.
Improved gas-liquid contact
The goal of improved gas-liquid contact is to remove elemental sulphur from the gas via the liquid where the sulphur can be more easily managed. Once in the liquid, it is important to keep any solids that form suspended in solution until they either dissolve or reach an appropriate point for removal from the system. The liquid load to the scrubber can be increased by using a high recirculation rate and packing with a large open area suitable for large liquid loads. High liquid loads in the caustic scrubber may provide the following benefits:
- Enhanced physical removal of sulphur from the gas (via increased gas-side pressure drop and eliminating gas bypass paths).
- Prevent accumulation of sulphur solids on packing surfaces by sweeping away existing solids and wetting the packing surface to eliminate places for condensation and crystallisation.
These methods are primarily effective via increased gas pressure drop, droplet/ particle impaction, and physical washing of surfaces. Therefore, submicron particles (solid particulate “dust” or liquid aerosols) are unlikely to be effectively managed by these approaches.
Removal of elemental sulphur solids
The dissolution of elemental sulphur into the caustic solution is the ideal approach to manage elemental sulphur and scrubbers in this service should ideally be designed and operated to facilitate dissolution.
However, if dissolution is not possible in the specific system (sulphur content is too high; temperatures limit reaction rates; system is built and cannot be modified to enhance dissolution; etc.), then it is important to manage the solids with minimal process interruption. Existing systems may be modified in order to implement some of these approaches.
Suspended elemental sulphur solids can be removed from the liquid phase in a variety of ways. The solids could be allowed to settle in a separate vessel and then be removed. A weir installed in the scrubber sump (see Fig. 2) to allow for overflow of a floating powder layer (if present) has been used in this service. Liquids could also be filtered to remove solids.
Liquid sprays could be considered to remove solids at different locations in the process (e.g. just above the gas entry in the scrubber, in the gas entry pipe itself, mist eliminator, or gas exit piping)12 .
Alternative equipment and process flow schemes
There are several other approaches that have been applied or considered for this application that have been presented in the literature12 − the following list provides an overview of a few:
- A venturi contactor (an eductor) with caustic as the motive fluid at a high circulation rate to provide the needed contact between the gas and liquid phases (also potentially serving as a motive device for the downstream scrubber). Venturi scrubbers are a known means of particulate removal and should reduce the particulate load to a downstream caustic scrubber.
- Proprietary commercial scrubbers have been designed to counter-currently contact the vent gas with caustic solution in a reverse jet nozzle that then separates in a wide open vessel (with chevron mist eliminator) to minimise places for sulphur solids to accumulate22 .
- Water scrubbing (in a tower or venturi) for particulate control prior to a caustic scrubber is also used; in Trimeric’s experience, this approach has mixed results in practice.
- Cross-flow (horizontal) scrubbing arrangements have been reported to withstand more than an order of magnitude more particulate than vertical counter-current scrubbers24 and have been used on vents from molten sulphur operations25 .
- Redundancy in the caustic scrubber design could be used to minimise downtime and allow cleaning. The extent of redundancy (fully redundant scrubber vs redundant components) will be an economic optimisation.
Operating strategies to mitigate plugging
There are also several operational changes that could be implemented to control the formation of solids or manage solids in the system. These strategies are reviewed in the following subsections.
Additives
Additional chemicals could be added to the scrubbing liquid to help wet elemental sulphur particles to enhance dissolution. Research has indicated that surfactants (e.g., sodium dodecyl sulphate, SDS) can increase the amount of elemental sulphur suspended in aqueous solution and enhance reactions by other mechanisms14, 26 . Examples of surfactant use in field applications include surfactants with caustic to remove sulphur solids in gas production wells9 and surfactant in scrubbing solution to resolve level measurement issues (sulphur accumulation at gas-liquid interface) in a sulphur melter offgas application18 . Other additives such as oxidisers and diesel oil have also been used16 .
Caustic temperature and strength
Temperature control of the caustic scrubbing system can be used to prevent or manage elemental sulphur in the system. High-temperature scrubbing could be used to prevent condensation of sulphur vapour and enhance the reaction rate of sulphur with the caustic solution. Controlling the temperature in different parts of the scrubber could be used to prevent the formation of aerosols and liquid sols (prevent rapid cooling of sulphur vapour in the scrubber) and control the growth of aerosols. Steam sparging could be used in specific areas (e.g., mist eliminators, column vapour inlet) to maintain localised higher temperatures with the added benefit of wetting surfaces. Trade-offs of high-temperature scrubbing must be considered carefully, however, as performance (ability to meet treatment specs) and cost of the scrubber (materials of construction) may be significantly impacted. Higher caustic concentrations will lead to enhanced reaction rates with elemental sulphur, improving dissolution. The caustic strength has the same challenge regarding column material selection as with higher temperature operations. If the system is operated with regular feed of fresh caustic to maintain original caustic strength, this may also help with dissolution of sulphur.
Exercising the unit
In other process systems where salt and solid sulphur plugging often occurs, ‘exercising’ the unit has been found useful to extend run times. An example of such a system where plugging has been experienced is a liquid redox sulphur recovery system (e.g., Sulferox, LO-CAT, Stretford). In this context, exercising the unit means varying flows, levels, and other operating conditions temporarily with the specific purpose of keeping the system clean. Control valves may be cycled, manual valves cycled, motor speeds changed, levels built and dropped with the goal of dislodging any material that is beginning to accumulate so that the material can be moved through the system to a point where it can be removed (or solubilised). In Trimeric’s experience, units that have implemented a regular system of ‘exercising’ the process have operated much longer than systems that had no such programme.
Cleaning the unit
If solids plug the system, then there are several approaches for cleaning it. The system could be opened and cleaned; however, this is a labour intensive process that could require significant down time. Other approaches have been applied in similar applications12 and could be adapted to molten sulphur vent scrubbing:
- Use of warm caustic (~140°F / 60°C) to remove sulphur solids. Applied in oil and gas and mining applications27 .
- Use of higher strength caustic for cleaning a unit. This approach has been used in other processes (e.g., in Stretford absorbers), including methods to clean while operating28 .
Materials compatibility should be reviewed for these approaches.
Managing upstream operations
Finally, the operation and monitoring of the upstream molten sulphur process may help with sulphur deposits as well. For example, a molten sulphur tank can be operated with reduced levels or lower temperatures to minimise the sulphur vapour pressure and reduce fog formation potential. The tank should also be regularly monitored for the presence of steam leaks in any internal coils, which is believed to cause additional elemental sulphur particulate to be present in the tank vapours.
Conclusions
Caustic scrubbing of molten sulphur vent gas streams has occurred with varying degrees of reliability due to the presence of elemental sulphur that can exist in several different forms (vapour, aerosol/fog, sols, and solids particulate). However, there are many design and operational modifications to enhance the performance of even the most difficult applications. Design changes can range from enhancing the gas/liquid contact with increased circulation rates, installing spray nozzles in different areas of the process, removing solids from the solution with filters or decanting floating solids, and using redundant or alternative contacting devices (dual contacting devices, piping, etc.). Operational changes can include the use of surfactants, elevated operating temperatures, and increased caustic strength. Understanding the chemistry and dissolving of the elemental sulphur into the caustic solution represents the ultimate mitigation technique, because the formation of solids is eliminated (or greatly reduced). The mechanisms for dissolving sulphur in caustic that are presented in this article can be used to aid in the development of a more robust caustic treating system for molten sulphur vent gas streams.
References






