Sulphur 411 Mar-Apr 2024
31 March 2024
Cobalt-molybdenum catalyst activation in low temperature TGUs
CLAUS TAIL GAS TREATING
Cobalt-molybdenum catalyst activation in low temperature TGUs
Part 2
Cobalt-molybdenum (CoMo) catalysts are integral components of tail gas units (TGUs), playing a vital role in reducing harmful sulphur dioxide (SO2 ) emissions arising from Claus sulphur recovery units. Effective activation of these catalysts is essential for their optimal performance. The consequence of sulphiding at low temperatures and atmospheric pressure in low temperature TGUs is to compromise effectiveness of catalyst activation. In the final part of this two-part article, Michael Huffmaster, Consultant, presents case study results using a discrete reactor model incorporating heat, mass transfer, and activation reaction kinetics to assess the impacts of these variables on in-bed temperature profile and activation effectiveness. Tailoring gas rate, composition, and temperature progression can achieve in-bed exotherms which improve CoMo catalyst activation effectiveness for low temperature tail gas units.
Discrete TGU catalyst bed model – activation simulation
A discrete reactor simulation embracing heat and mass transfer and activation kinetics enables evaluation of process alternatives within constraints. Procedures to improve catalyst activation are assessed with this simulation tool as well as in a case study, evaluating variables and conditions and developing recommended enhanced practices.
The core of the simulation tool is kinetics, representing rate of conversion of metal oxides to respective sulphides, and heat transfer, to determine distribution of heat of reaction between the catalyst and the gas and to generate an in-bed temperature profile. Mass transfer is included, but does not present a controlling influence on sulphiding conditions. The approach taken was fitting kinetics to actual activation based primarily on observed outcomes and measurements, as well as data from the field. Experimental data from a 2019 study of activation at low temperature tail gas conditions are the basis for kinetics derived to represent the degree of activation achieved9 .
This semi-empirical representation embraces the varied, multiple pathways and intermediates, not yet emerged as definitive, but using outcomes that have been measured, especially for low temperature. A formal reaction set lacks sufficient characterisation to apply. Having measurements and parametric variances to assess sensitivities for key variables is very useful.
The model framework is a first order rate model which proved sufficient to represent the data and accommodate the following parameters:
Rate of reaction for metal (oxide) with sulphiding media proportional to metal mass (area); cobalt and molybdenum are combined for “average metal” heat of reaction and H2 S and H2 consumption:
- knowledge that mixed phase intermediates are likely, e.g., oxy sulphide;
- division of metal into three levels of reactivity/accessibility using crystallites or pillow shaped platelets visualisation – stacked layers
– restricted – interior (or occluded layers) with diffusional resistance
– difficult – metals interacted with substrate, higher activation energy;
- reaction kinetic coefficient is proportional to H2 S partial pressures;
- reaction kinetic coefficient is proportional to square root (H2 partial pressure and adsorption coefficient); hydrogen consumption proportional to H2 S reacted;
- reaction kinetics fitted with Arrhenius relationship for temperature dependence;
- crystallisation not explicit, approximated as with overall sulphidation.
Water has minor impact on reaction rate. Heat effects and heat transfer
- heat from reaction proportional to H2 S consumption/metals reacted;
- heat of adsorption (desorption) for water; considered as chemisorption, catalyst water loading in equilibrium with gas phase Heat transfer and mass transfer based on fluid/material properties, Eckert packed bed correlations, and Colburn analogy;
- effectiveness factors near unity; film fluxes are relatively small and Knudson diffusion resistance is small.
The vision of crystallite sizes used is based on a rationale of what will fit, but not obstruct, pores of alumina, as particles, not a simple mono-layer. The number of “metal oxide atomic units” are on the order of 50 to 200 per nanoparticle with one to three layers stacking; exposed edge and surface ratio to total crystallite, is 100% for single layer and 63% for average 2.6 layers. This size distribution agrees with SEM and TEM and other data representing the arrangement of MoO3 and CoO on the surface of alumina and in pores.
The simulation model is two dimensional: one in space and one in time. Plug flow is assumed. It is solved in 100 discrete steps along the gas flow path, based on sulphiding gas flow, composition, and conditions. The reactor bed is then moved ahead to the next time increment and the gas flow path solved. The reactor bed is moved through ~100 time steps to the completion the activation. A reactor profile is generated for temperature and gas composition and for catalyst metals, water, and temperature for each step through the reactor and each step through time.
Tail gas catalyst activation at low temperature is recognised at the margin of the temperature and hydrogen partial pressure for effective activation. Application of information from recently published work in concert with TGU activation studies and field experience can lead to measures which can improve low temperature activation.
Heats of reaction are applied to metal sulphided, using ΔH from thermodynamics, proportioning along reaction pathway as the fraction of metal mass converted. Thermodynamics and kinetics for intermediates are not explicitly known. Kinetics are set to represent that initial reactions steps are vigorous (based on observed plant exotherms).
Experiments are almost always in temperature-controlled settings, whereas plant scale sulphiding is adiabatic. In plant settings, exotherms driven by heat from reaction must be managed. The heat of reaction is determined based on thermodynamic database for defined substances. Density functional theory suggests even energy distribution across intermediates for sulphur-oxygen exchange8,15,16 .
The model provides bed temperature profiles and degree of activation as a function of sulphiding parameters, including influence of gas temperature and rate, concentration of H2 S, H2 , and H2 O, as well as time of duration for activation. Case study results incorporate heat and mass transfer and activation reaction kinetics to assess the impacts of these variables.
Procedures
Catalyst activation procedures for gas phase HDS and traditional tail gas are similar. Initial steps are almost the same even for low temperature tail gas. Low temperature activation only achievies 240°C and does not raise the catalyst to the traditional 315°C temperature finish.
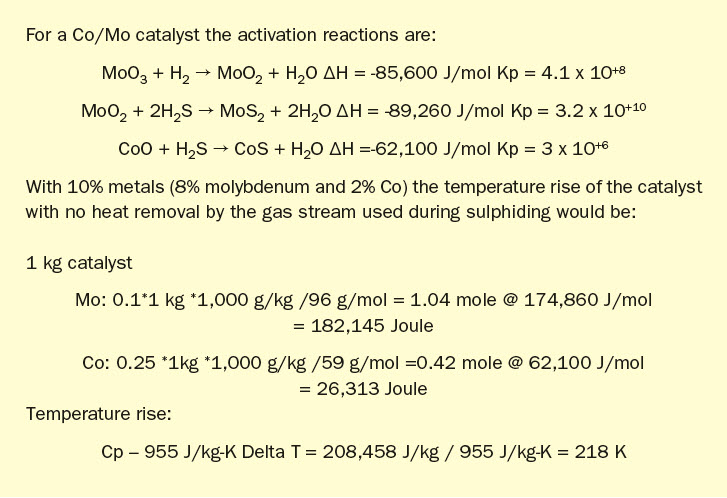
Tail gas procedures are conducted at atmospheric pressure and begun at 180 to 200°C. Sulphiding is initiated cautiously, with H2 S and H2 , and with control, to manage temperature rise from vigorous initial metal reactivity. A reference sulphiding procedure is presented, based on a 2007 presentation at Brimstone by Steve Massie17 .
Traditional sulphiding procedure:
- Purge reactor to remove oxygen and establish (re)circulation at ~500 aghsv (320 nghsv)
- Ramp temperature to 130°C at 50°C/ hr; hold for 1 to 2 hours to remove water from catalyst; water dew point should be controlled to 40°C in recirculation loop, removing water in quench column.
- Ramp temperature to 200°C at 50°C / hr: During this time hydrogen may reach 2% if a RGG is used for heating, firing at 95% stoichiometry.
- Introduce sulphiding gas: add H2 S at 1% and H2 at 4%. Monitor bed for exotherms, not to exceed 30°C;
- Ramp temperature to 240°C at 20°C/ hr and hold for 1 hour: Ensure H2 S out of bed is 3,000 ppm before increasing temperature further.
- Ramp temperature to 315 °C at 20°C/ hr; increase sulphiding gas to 1.5% H2 S; hold for 3 hour heat soak.
- Total 12 hours at sulphiding conditions; once completed reactor can be cooled to 250°C and placed in service or standby.
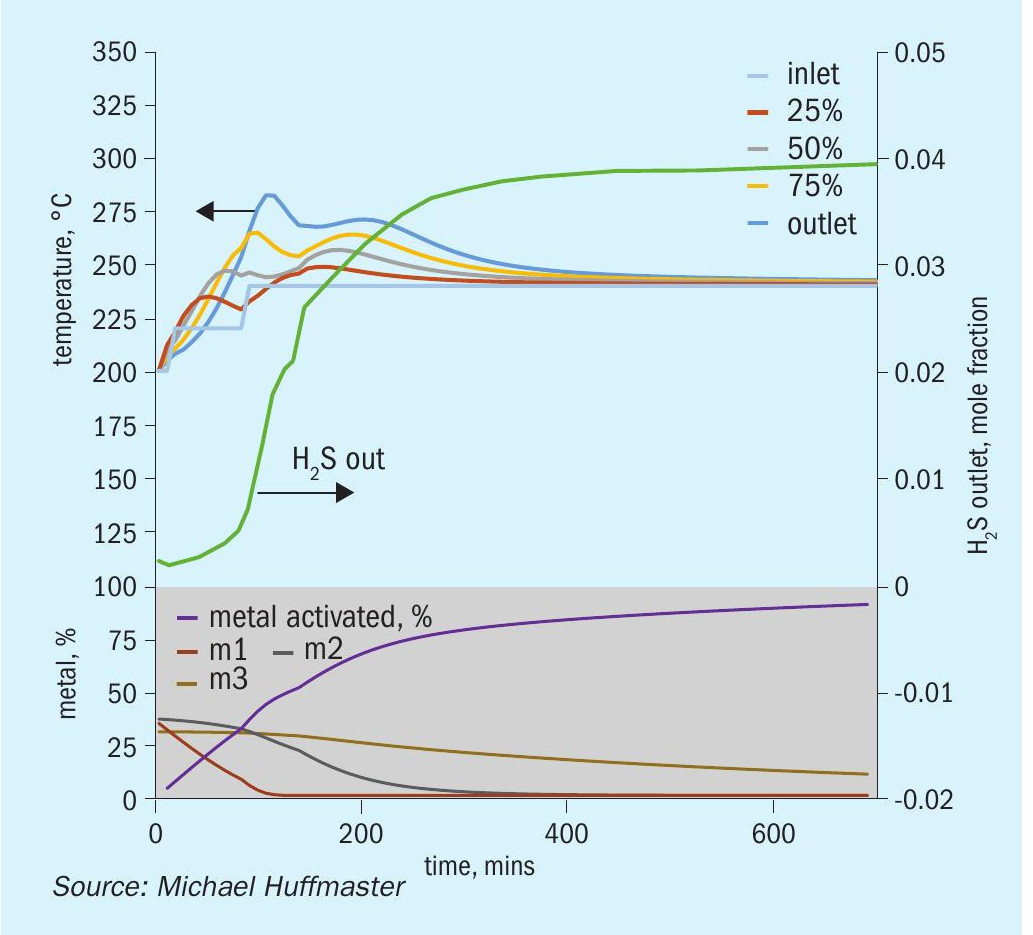
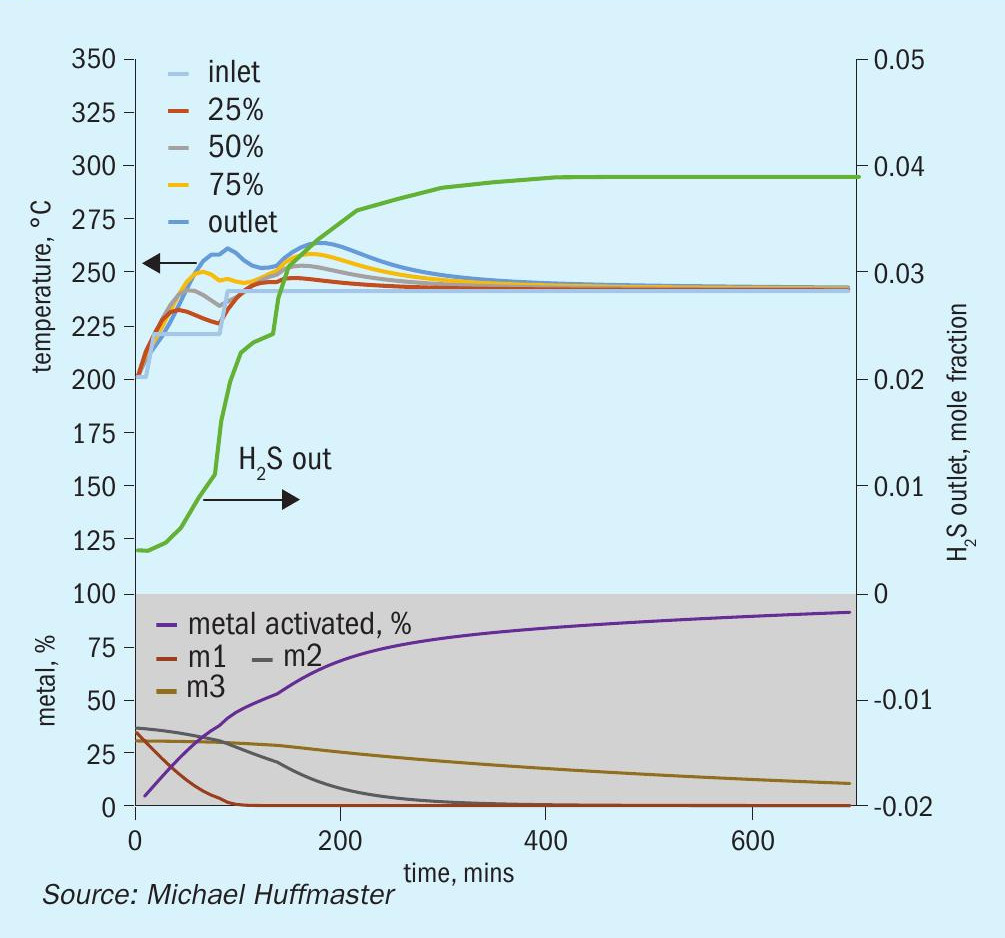
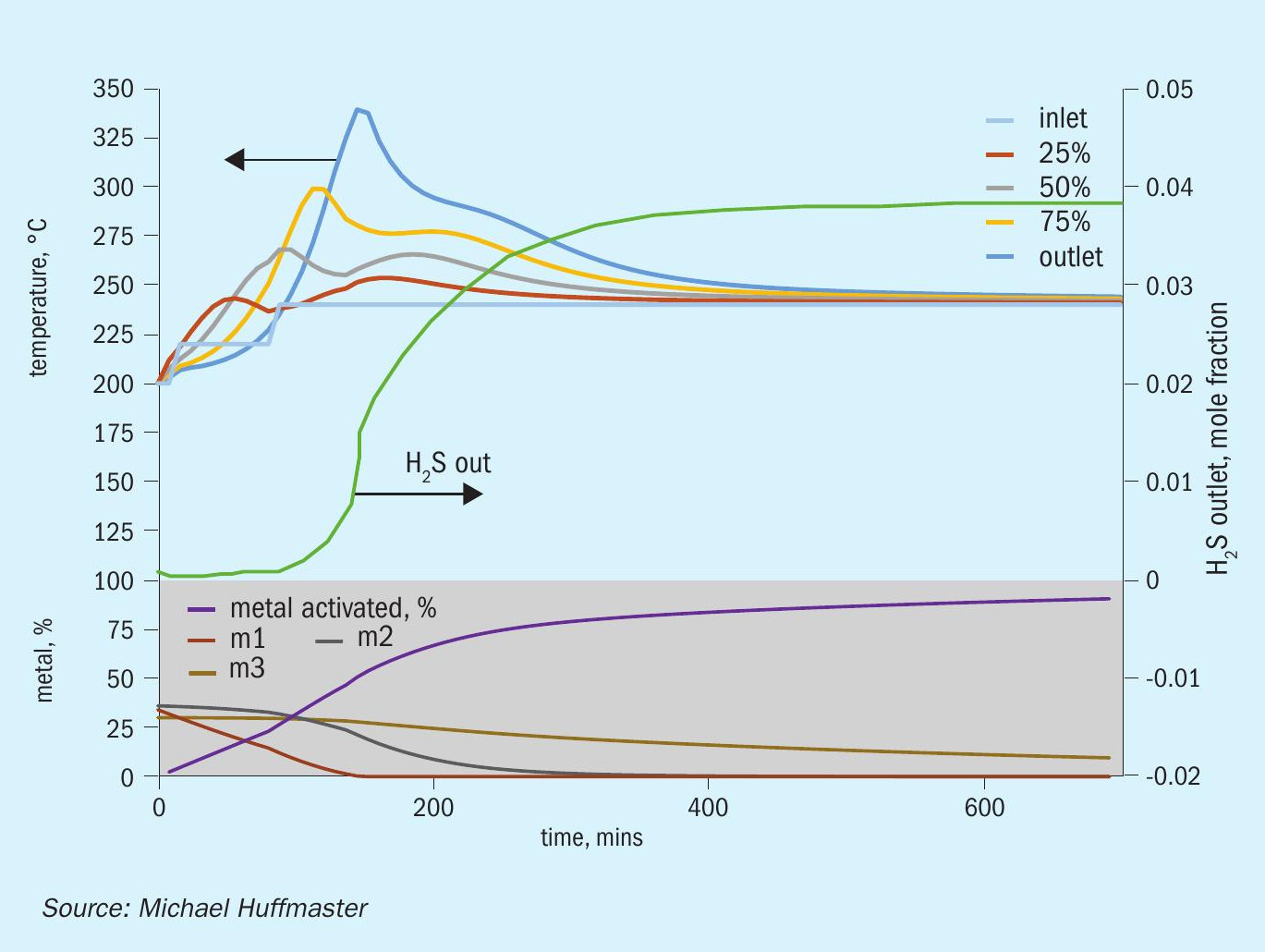
The graphic in Fig. 6 depicts this procedure and low temperature adaptation. Fig. 7 presents activation model predicted temperature profile and activation effectiveness for the traditional sulphiding procedure and Fig. 8 presents activation model results for low temperature.
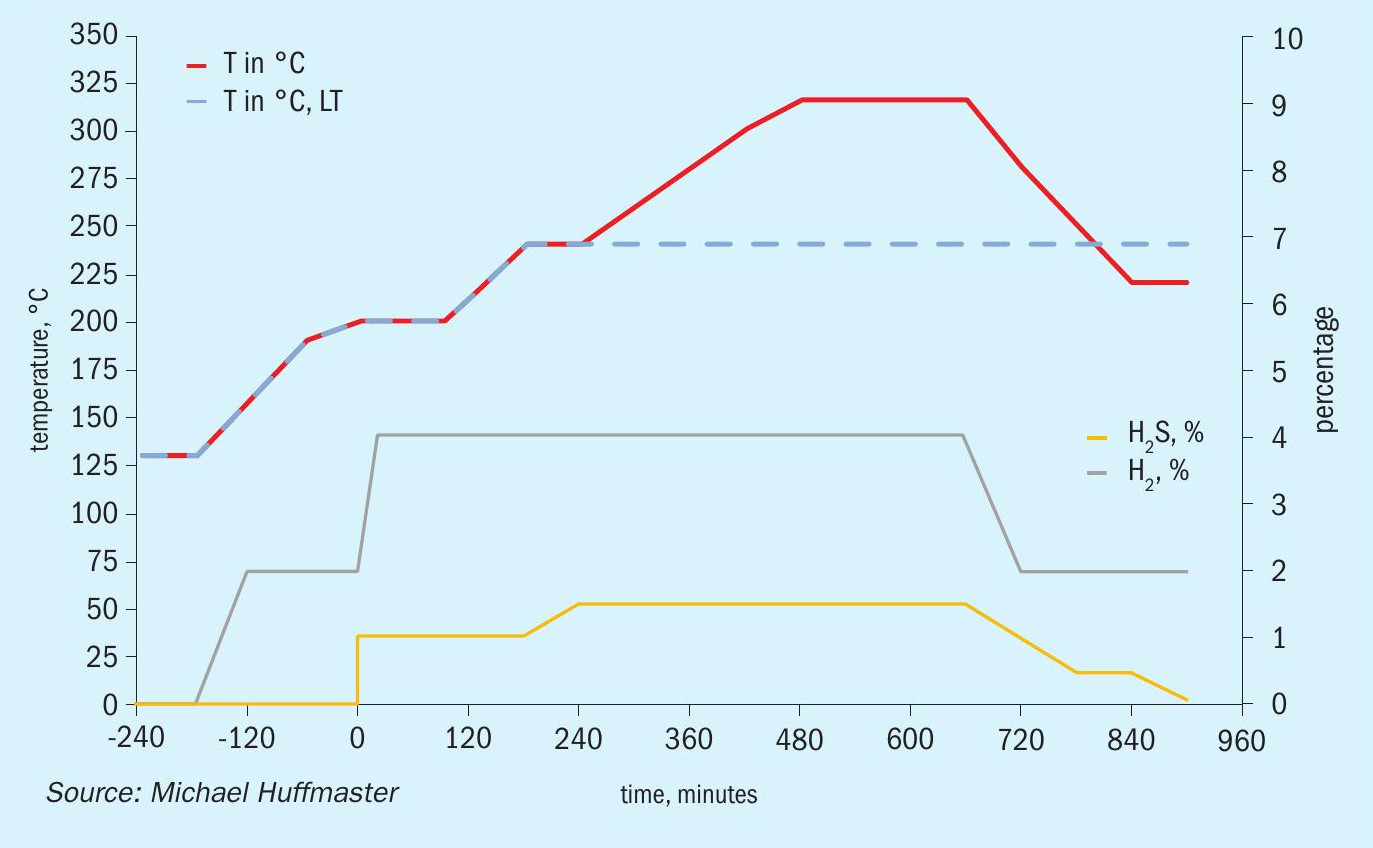
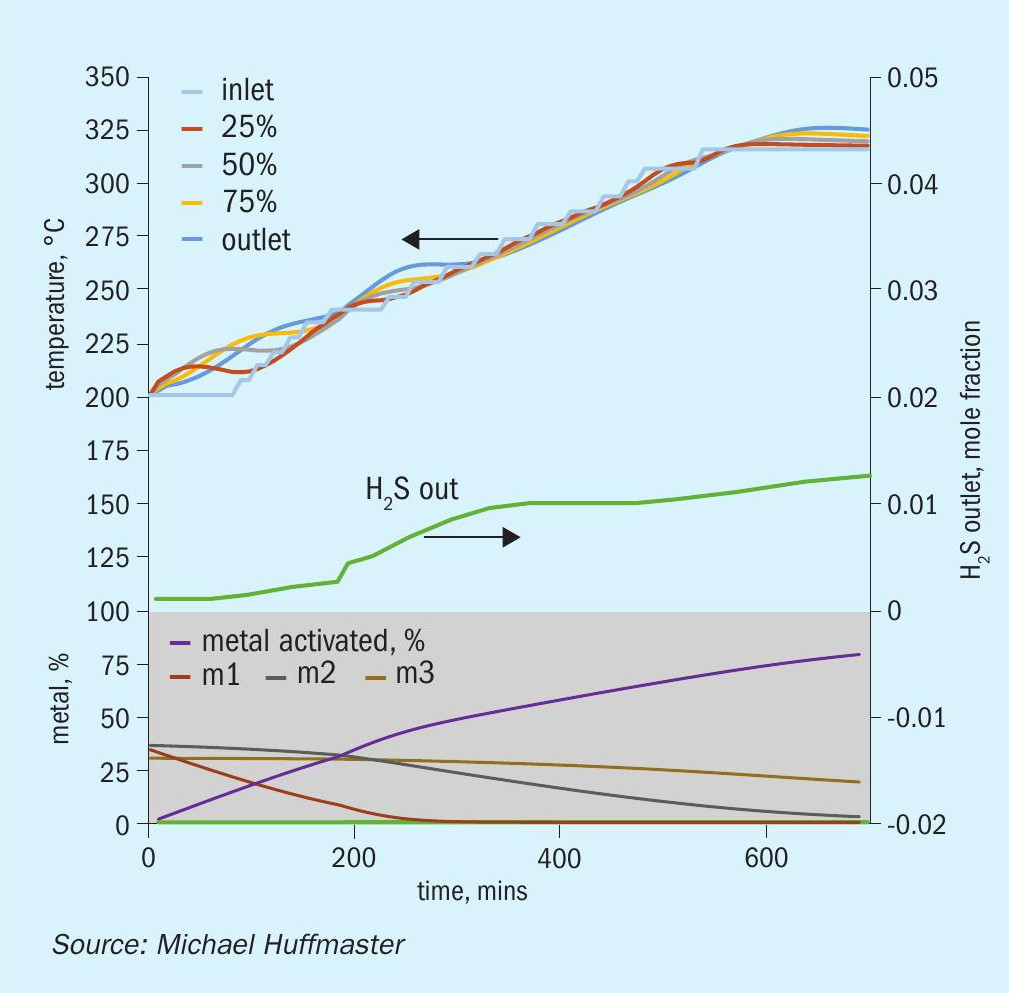
Typical low temperature TGU sulphiding follows this procedure except a final temperature and soak is at 240°C; this is indicated as a dashed line in the graphic.
Activation steps (noted for activation pathways from part 1):
- Initial sulphiding below 200°C – formation of oxy sulphides and MoS3 morphs, MoS2 and MoO2 begins; exotherms create temperature waves in the bed.
- Temperatures are raised with sulphur uptake and production of MoS2 and conversion of oxy sulphides.
- At 240°C Sulphur uptake is expected to be 50 to 75%. Hold to verify H2 S is penetrating the entire bed to ensure cobalt in the lower bed is not reduced by being in a hydrogen atmosphere without H2 S above 200°C.
- Temperatures are ramped to the critical 260°C transition. Transformation and completion of sulphiding follows, heating on at a controlled rate to 315°C final bed temperatures for another hold point to anneal and crystalise the MoS2 nanoparticles.
Cautions and concerns
There are a few concerns to address regarding activation. The foremost concern should be for temperature in excess of 325°C due to strong exotherms. This peril arises during initial sulphiding period with vigorous reactions. If H2 S concentration is too high a lot of heat is released or if gas rates are low, insufficient heat is transported from the catalyst particles. This can cause temperature stacking and accelerated reactions at the elevated temperatures. Increasing the gas rate or reducing the H2 S will calm strong exotherms.
Another concern should be reduction of cobalt to metal. This agglomerates cobalt and reduces promotion and results in low activity for the catalyst. It is caused by exposure of catalyst to H2 at temperatures above 200°C without the presence of H2 S. The risk during sulphiding is H2 S starvation, consuming H2 S in the upper bed with low concentrations of H2 S in the lower bed.
A similar and related concern is reduction of molybdenum; however, sulphiding conditions are not sufficiently strong to reduce MoO3 . Hydrogen alone will not reduce MoO3 below 400 to 500°C10 nor MoO2 to metal until 1,100°C [NIST]. Moreover, water inhibits these reduction reactions.
Exposure of catalyst to H2 S in the absence of H2 could potentially form MoS3 . This is a less active catalyst which needs to be reduced by H2 or decomposed at 315°C.
Exposure of sulphide catalyst to hydrogen without H2 S has a risk of stripping sulphur. This is largely mitigated for TGU because of low partial pressures of hydrogen, especially if temperatures are below 250°C. Addition of small amounts of H2 S offer further protection, with 100 ppm being quite sufficient.
Finally, there is concern of exposure to oxygen. If oxygen is present during sulphiding it will inhibit conversion of oxides to sulphide. Exposure of sulphide catalyst risks sulphation of support and oxidation of active metals, which can severely damage the catalyst.
Low temperature consequences
Low temperature tail gas diverges from traditional tail gas because bed inlet temperatures may be limited to 240°C and with restrained temperature ramp rate bed temperatures may not reach 250°C. Activation at maximum temperatures of 250°C has notable consequences on the activity potential of cobalt-molybdenum catalysts.
The consequence of not going through the 250 to 260°C transition may leave a significant fraction of metals not converted to sulphide. There will be higher valence molybdenum in oxy sulphide, MoO2 and potentially MoS3 . Spectrographic tracking of activation revealed Mo+IV increased from 17 to 70% at this transition7 . MoS2 formed is amorphous and less effective as a catalyst.
Furthermore, a temperature above 260°C drives the transformation of amorphous MoS2 to crystal slabs. At lower temperatures, the mobility of atoms and molecules is restricted, potentially affecting the dispersion and structural transformation of the catalyst metals. In particular, nucleation and crystallisation of MoS2 may not occur and, absent MoS2 slab, cobalt attachment at edge features and promotion may be reduced. The activation process is not as comprehensive as at higher temperatures, resulting in suboptimal active site formation and reduced catalytic performance. Thus, low temperature activations produce weak catalytic kinetic activity.
It is known that low temperature activation is partially successful as catalysts do function, albeit in diminished capacity, and units perform, although maybe at reduce catalyst life cycle. Thus, we know some formation of active slabs is occurring. What we want to know is if the activation step be managed to enhance and improve activation.
The critical factor is to achieve 260°C, or perhaps to target 270°C which allows some margin for reported transition temperature and offers allowance for low hydrogen partial pressures in tail gas applications. This should initiate the vital transition of amorphous MoS2 and seeding nucleation sites for MoS2 nanoparticles. Time is necessary for transformation of amorphous MoS2 (coordinated with 4 sulphur) to MoS2 crystalline (Mo coordinated with 6 sulphur). Also consider that nucleation in principle needs to occur in each independent nanoparticle.
Extending time in sulphiding conditions improves activity, so the indication is more slab development/promotion occurs. Although it may not be as effective as traditional temperatures, it is an enhancement which can be applied in LT TGUs. With transition achieved, annealing might proceed at lower temperatures. The rate of crystallisation is slower at lower temperatures, but with nucleation initiated, perhaps an acceptable level of slab formation will occur with extended time.
Case study examples
An activation process model for the outlined activation procedure is presented in the form of charts in Fig. 9 through Fig. 15. Several variations or adjustments to temperature ramping, gas rate and composition are assessed. A best case recommendation is offered and parametric adjustments of variables are made for sensitivity.
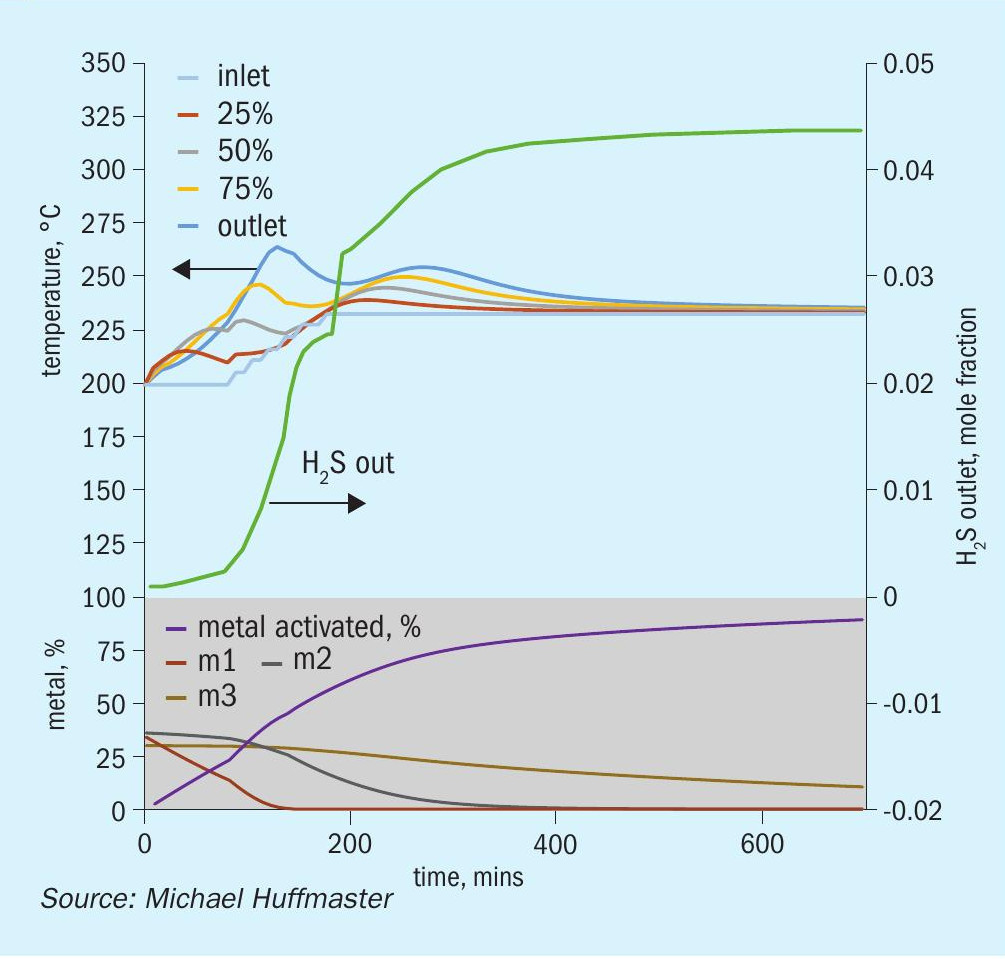
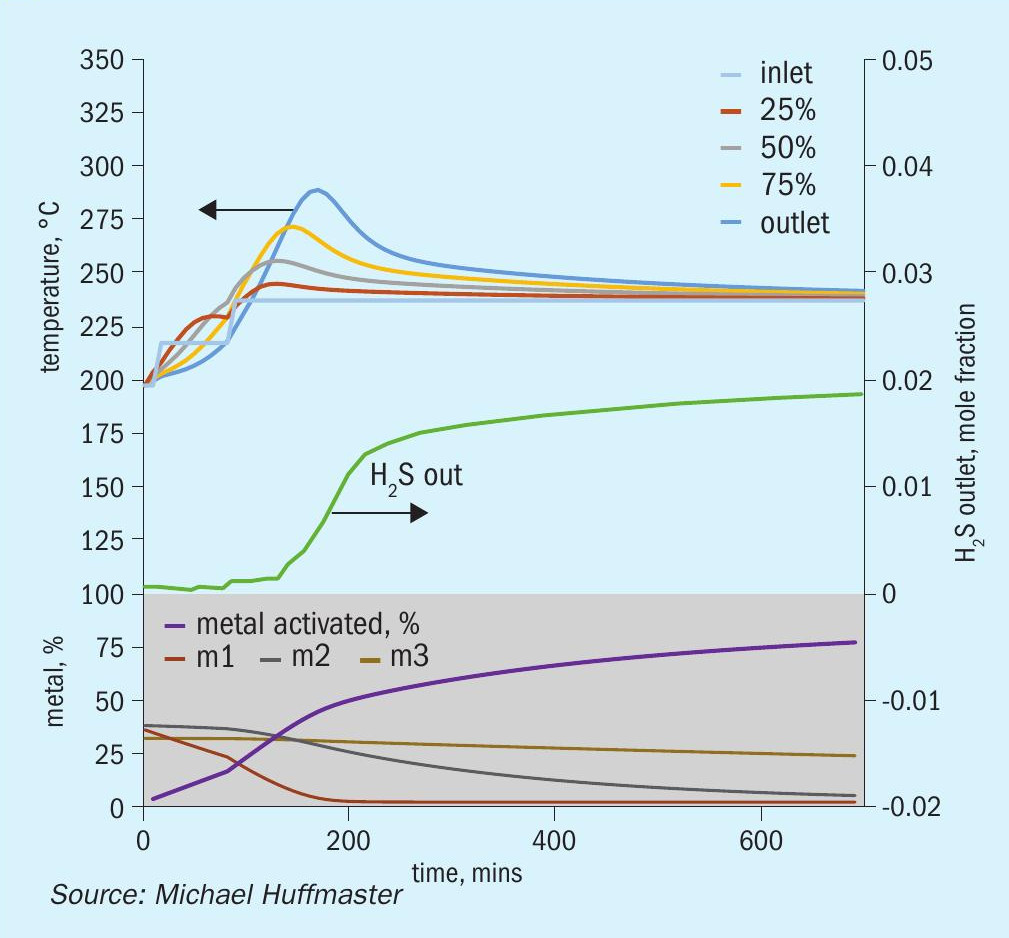
The charts each include temperatures for inlet gas and temperatures at bed quarter points including bed outlet across 12 hours, roughly the duration of a sulphiding procedure. Low temperature procedure duration would be extended for longer heat soak to further activation.
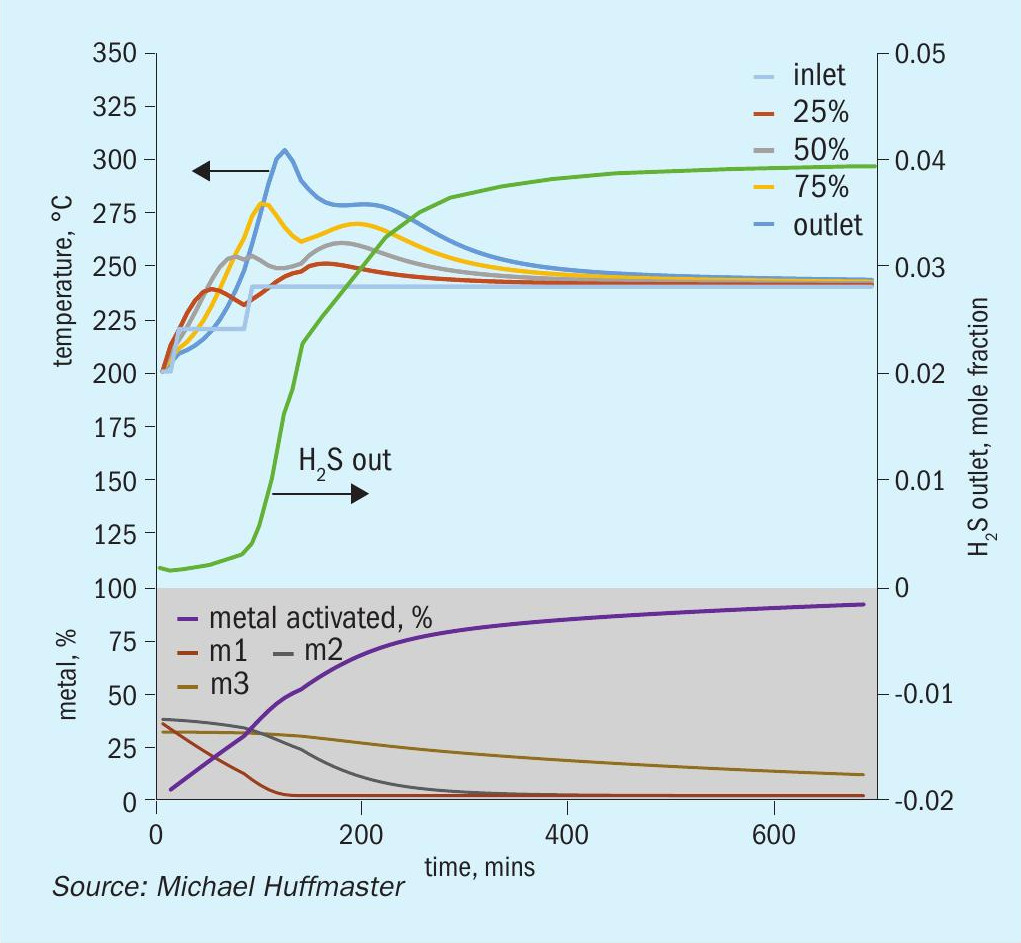
Also tracked are outlet H2 S concentration, percentage of metal converted/activated and percentage of vigorous, moderate and difficult metal fractions.
The calculations are based on an alumina supported CoMo catalyst with 2.5 wt-% cobalt and 10 wt-% molybdenum in oxidic form. This is an illustrative catalyst, so can be considered either spherical or extrudate, although physical properties are more typical for high pore volume extrudate.
Reference sulphiding: procedure 200°C starting temperature, (re)circulation of sulphiding gas at 320 nghsv, 40°C water vapour for 10% water, 1.0 to 1.5% H2 S, 4% H2 , 15°C per hour ramp, 240°C hold for LT finish.
The rate of initial reactions is managed in part by the concentration of the sulphiding agent, e.g., H2 S and H2 concentration. Increasing H2 S concentration initially and then stepping higher enhances bed temperatures. Reducing H2 initially moderates reaction followed by stepping higher to drive more complete activation.
The rate of temperature ramping impacts bed temperature, as slow ramp rates allow more heat to depart with the gas. The step from 200 to 240°C accomplished in 1.5 hours increases bed temperatures, with a caution that gas rates must be sufficient.
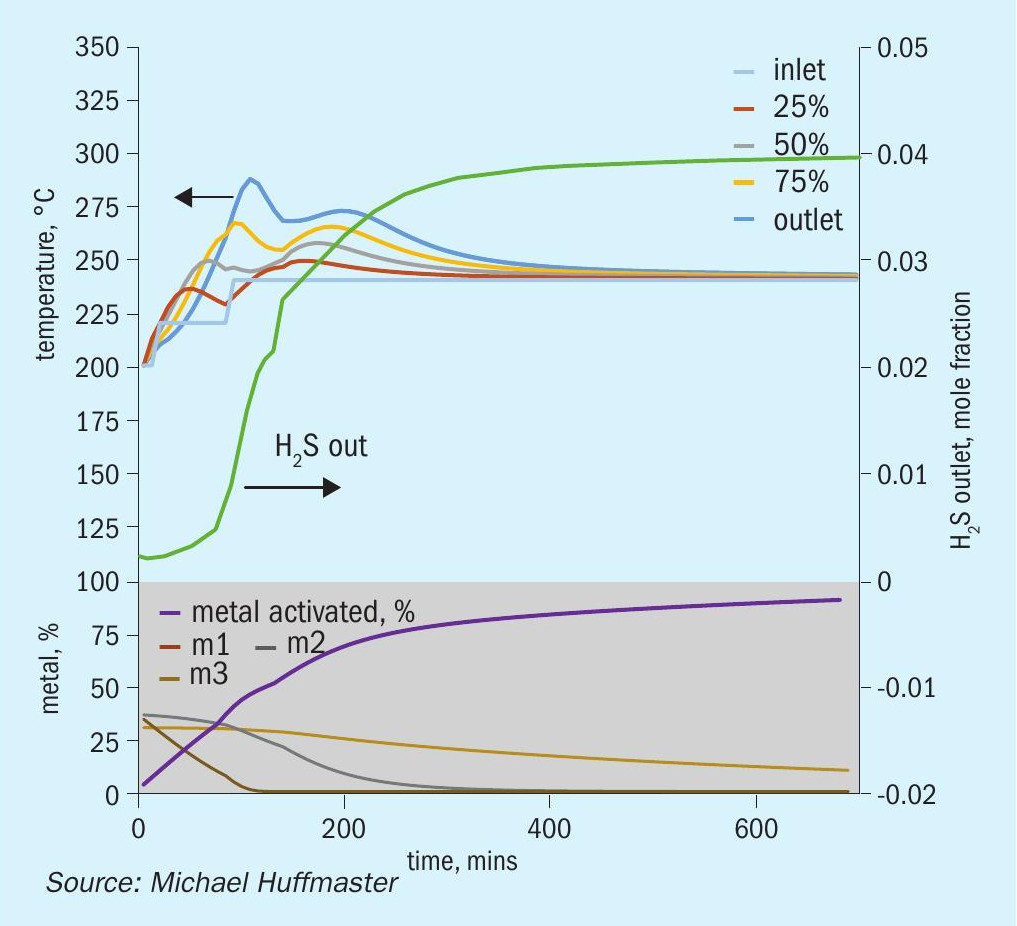
A major control is gas rate. Lower gas rates increase bed temperatures but below 240 nghsv risk excessive exotherms. Higher gas rates, above 600 nghsv, suppress exotherms and elevated water levels dampen exotherms.
Discussion
A critical aspect of activation procedures is managing exotherm in the bed. Energy released by sulphiding reactions heat the catalyst and the sulphiding gas.
Gas rates traditionally used in the range of 1/4 to 1/3 operating design gas rate (280 to 360 nghsv) proved adequate to control bed temperature, and H2 S concentrations were set to reduce risk of strong exotherms. However, very low H2 S values are often found at the bed outlet, especially when H2 S is held to low levels. The level of 1% H2 S typically applied causes starvation in the lower bed, putting catalyst in the lower portion of the bed at risk of damage and impairment from reducing cobalt.
Development of low temperature catalysts achieved stronger kinetic activity at low temperatures and also increased metal levels to provide overall similar activity to that of more conventional catalysts. With the increase in metals, the magnitude of exotherms is amplified.
A facet of increased exotherm magnitude is the appeal of utilising this heat to raise bed temperatures during sulphiding. This could provide a means to improve the low temperature activation challenge, but excessive exotherms must be avoided as they can damage catalysts. Managing exotherm magnitude is important because of the risk it can self-reinforce or run-away. As the temperature of the bed is increased, the activation reactions are more vigorous and proceed faster which releases even more heat. Temperature stacking comes in with heat released in the bed raising the bed temperatures further along in the flow path. By adjusting (increasing) gas rates the exotherm may be managed.
A further consideration and challenge is the need to have most of the initial or fast reactions completed before moving to elevated temperatures. This is a consideration for preventing excessive temperature. Also, the initial sulphiding steps need to be mostly completed, forming MoS2 , before the 240 to 260°C transition which initiates nucleation of the MoS2 .
The ability of the metal sulphiding reactions to increase bed temperatures is mostly from the initial vigorous reactions. The reactions which follow are kinetically limited or have lower energy release, limited in ability to raise bed temperature in either case. The opportunity to use the higher metal levels of low temperature catalysts to raise bed temperatures requires finesse to have most of the initial high energy reactions completed before moving to higher temperatures.
The critical factor is to achieve a 240°C to 260°C transition temperature and 260°C or a target of 270°C in the bed for transformation. Although it may not be as effective as traditional temperatures, it is an enhancement which can be applied in LT TGUs. The rate of crystallisation is slower at lower temperatures, but with nucleation initiated, perhaps an acceptable level of slab formation will occur with extended time.
Options for plant owners
Plant owners can consider improvements to existing facilities and enhancing plant specifications to address activation requirements. Areas of focus should be process information and equipment constraints. These include:
- managing activation to utilise heat of sulphiding to achieve bed temperatures of 270 to 315°C:
– to achieve a temperature above 260°C transition, one hour or preferably longer
– to increase H2 S concentration of 2.5% initial to 5% during sulphiding with gas rate set appropriately to control exotherms;
- placing more thermocouples in one zone of bed to provide temperature tracking on a fine grid of 10 cm;
- setting specification for plant design that addresses minimum activation requirements:
– minimum specifications sufficient for effective sulphiding, e.g., 275°C, preferably 300°C
– creative utilisation of supplemental heating designed just for catalyst activation
– including provision of proper supply of acid gas and hydrogen
– providing proper metering of streams used activation, e.g., hydrogen, acid gas (H2 S), and recycle gas;
- employing pre-activated catalyst;
- utilising very high activity catalyst and accept partial activation;
- changing catalyst more frequently to avoid running at low activity of aged catalysts.
Conclusions and recommendations
- Low temperature TGUs often are constrained to temperatures in the range of 220 to 240°C.
- Lower temperature prolongs sulphidation; activity may be as low as 2/3 of full.
- Advancement in low temperature activation work incorporates published research on activation mechanisms and identifies pathways.
- Key sulphiding conditions identified are:
– transition at 240 to 260°C, critical for molybdenum reduction,
– transformation requires 260°C, seeding nuclei needed to initiate crystallisation.
- Modelling to represent sulphiding kinetics with heat and mass transfer for adiabatic conditions provides bed thermal profiles for assessment of activation procedures.
- Initial vigorous reaction must be managed to avoid excessive exotherms and H2 S starvation.
- Sulphiding procedure enhancement opportunities for low temperature TGUs are:
– gas rate is major control, targeting 275 to 320 nghsv.
– raise initiation temperature and accelerated ramping.
– increasing hydrogen partial pressure; recommend 5% minimum and prefer 10%.
– increasing H2 S partial pressure; initiation at 1 to 2%; ramp to 4 or 5%.
- Extending time at 240°C improves activation, but does not achieve activity of traditional 315°C temperatures.
- Operations has options to improve sulphiding:
– set design specifications for effective sulphiding.
– employ pre-activated catalyst.
– utilise very high activity catalyst.
– change catalyst more frequently.
- Improving low temperature catalyst activation procedures enhances catalyst activity in the TGU reactor achieves low sulphur emission and environmental compliance.
Future work
Future work is needed:
- to develop thermochemical properties for reaction intermediates;
- to provide additional information on minimum effective H2 partial pressure;
- to provide field verification of amended procedures and model predictions.
References





